Abstract
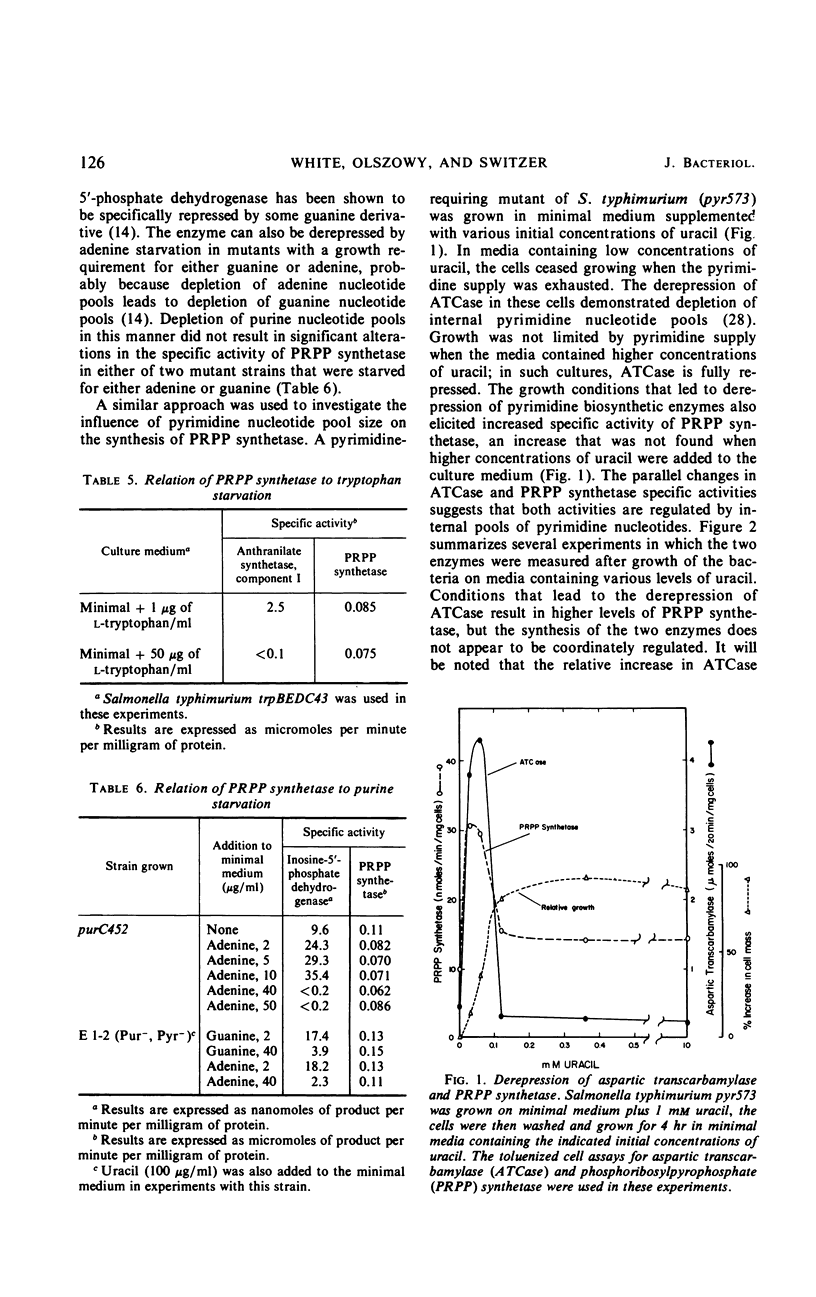
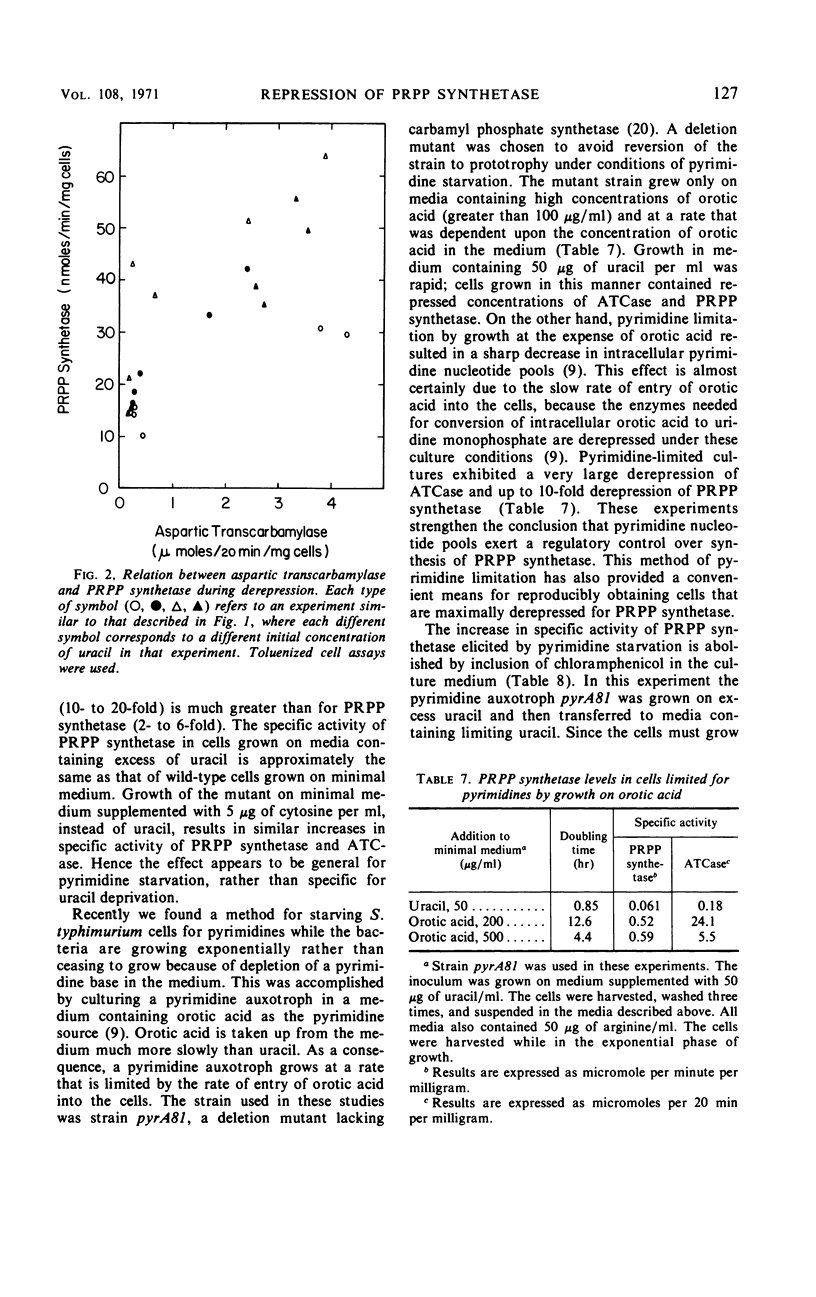
Phosphoribosylpyrophosphate (PRPP) synthetase participates in the biosynthesis in bacteria of purine nucleotides, pyrimidine nucleotides, tryptophan, and histidine. The regulation of the synthesis of PRPP synthetase in Salmonella typhimurium was studied. Addition of end products to the growth medium, singly or in combination, resulted in small decreases in the specific activity of PRPP synthetase, but levels of the enzyme were never decreased to less than half of those found when the bacteria were grown on minimal medium. Growth of the bacteria on several different carbon sources or starvation for phosphate had little effect on the specific activity of PRPP synthetase. Over-production of histidine in a histidine regulatory mutant, which would be expected to result in a depletion of intracellular PRPP pools, did not alter PRPP synthetase specific activity. PRPP synthetase levels were examined in auxotrophic strains of S. typhimurium that had been starved for the end products of PRPP. In each case derepression of an enzyme in the biosynthetic pathway for the limiting end product was demonstrated. However, only alterations in the levels of pyrimidine bases in the culture medium brought about derepression and repression of PRPP synthetase. Excess pyrimidines do not completely repress the enzyme. Deprivation of exponentially growing cells for pyrimidines by growth of an auxotrophic mutant on media containing orotic acid, which enters the cells slowly, resulted in a 10-fold derepression of PRPP synthetase. Derepression of PRPP synthetase during uracil starvation was prevented by chloramphenicol. The PRPP synthetase activities of extracts from repressed and derepressed cells responded in identical fashion to heat inactivation, cellulose acetate electrophoresis at several pH values, and in kinetic experiments.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- AMES B. N., MARTIN R. G., GARRY B. J. The first step of histidine biosynthesis. J Biol Chem. 1961 Jul;236:2019–2026. [PubMed] [Google Scholar]
- Baker T. I., Crawford I. P. Anthranilate synthetase. Partial purification and some kinetic studies on the enzyme from Escherichia coli. J Biol Chem. 1966 Dec 10;241(23):5577–5584. [PubMed] [Google Scholar]
- Bauerle R. H., Margolin P. A multifunctional enzyme complex in the tryptophan pathway of Salmonella typhimurium: comparison of polarity and pseudopolarity mutations. Cold Spring Harb Symp Quant Biol. 1966;31:203–214. doi: 10.1101/sqb.1966.031.01.028. [DOI] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P. Regulation of branched biosynthetic pathways in bacteria. Science. 1969 Aug 8;165(3893):556–562. doi: 10.1126/science.165.3893.556. [DOI] [PubMed] [Google Scholar]
- Dennis P. P., Herman R. K. Pyrimidine pools and macromolecular composition of pyrimidine-limited Escherichia coli. J Bacteriol. 1970 Apr;102(1):118–123. doi: 10.1128/jb.102.1.118-123.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., WATSON J. A., HOFFEE P. A. The glucose effect and the relationship between glucose permease, acid phosphatase, and glucose resistance. Cold Spring Harb Symp Quant Biol. 1961;26:261–276. doi: 10.1101/sqb.1961.026.01.033. [DOI] [PubMed] [Google Scholar]
- FREUNDLICH M., BURNS R. O., UMBARGER H. E. Control of isoleucine, valine, and leucine biosynthesis. I. Multivalent repression. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1804–1808. doi: 10.1073/pnas.48.10.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN S. C., BUCHANAN J. M. Biosynthesis of the purines. XXI. 5-Phosphoribosylpyrophosphate amidotransferase. J Biol Chem. 1958 Aug;233(2):451–455. [PubMed] [Google Scholar]
- Irr J., Gallant J. The control of ribonucleic acid synthesis in Escherichia coli. II. Stringent control of energy metabolism. J Biol Chem. 1969 Apr 25;244(8):2233–2239. [PubMed] [Google Scholar]
- LEVIN A. P., MAGASANIK B. The effect of purines on the formation of two enzymes involved in purine biosynthesis. J Biol Chem. 1961 Jan;236:184–188. [PubMed] [Google Scholar]
- LIEBERMAN I., KORNBERG A., SIMMS E. S. Enzymatic synthesis of pyrimidine nucleotides; orotidine-5'-phosphate and uridine-5'-phosphate. J Biol Chem. 1955 Jul;215(1):403–451. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- Neuhard J., Ingraham J. Mutants of Salmonella typhimurium requiring cytidine for growth. J Bacteriol. 1968 Jun;95(6):2431–2433. doi: 10.1128/jb.95.6.2431-2433.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREISS J., HANDLER P. Biosynthesis of diphosphopyridine nucleotide. II. Enzymatic aspects. J Biol Chem. 1958 Aug;233(2):493–500. [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Shapiro B. M., Kingdon H. S., Woolfolk C. A., Hubbard J. S. Cellular regulation of glutamine synthetase activity in Escherichia coli. Adv Enzyme Regul. 1968;6:257–289. doi: 10.1016/0065-2571(68)90017-4. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R. The role of multiple enzymes in the regulation of branched metabolic pathways. Ann N Y Acad Sci. 1968 Jun 14;151(1):516–530. doi: 10.1111/j.1749-6632.1968.tb11911.x. [DOI] [PubMed] [Google Scholar]
- Switzer R. L. Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem. 1969 Jun 10;244(11):2854–2863. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- YANOFSKY C. Indole-3-glycerol phosphate, an intermediate in the biosynthesis of indole. Biochim Biophys Acta. 1956 May;20(2):438–439. doi: 10.1016/0006-3002(56)90332-8. [DOI] [PubMed] [Google Scholar]
- YATES R. A., PARDEE A. B. Control by uracil of formation of enzymes required for orotate synthesis. J Biol Chem. 1957 Aug;227(2):677–692. [PubMed] [Google Scholar]


