Abstract
Cell-free extracts of Bacillus megaterium form β-cyanoalanine (β-CNA)-14C from Na14CN and l-cysteine, O-acetyl-l-serine or, to a lesser extent, l-serine. However, the presence of cyanide in the growth medium does not increase the capacity of cell extracts to catalyze the formation of β-CNA from cysteine and cyanide. The formation of β-CNA is readily detected in extracts of cells grown in synthetic media with sulfate or l-djenkolic acid as sulfur sources; such cells also exhibit an increased ability to form cysteine when compared with cells grown on cysteine as the sulfur source. β-CNA formation could not be detected in extracts of cells grown on cysteine as the sulfur source. A 40-fold purification of the O-acetyl-serine sulfhydrylase resulted in the co-purification of the β-CNA-forming activity. The sulfhydrylase and the β-CNA-forming activity co-chromatographed on diethyl-aminoethyl cellulose and Sephadex G-100.
Full text
PDF

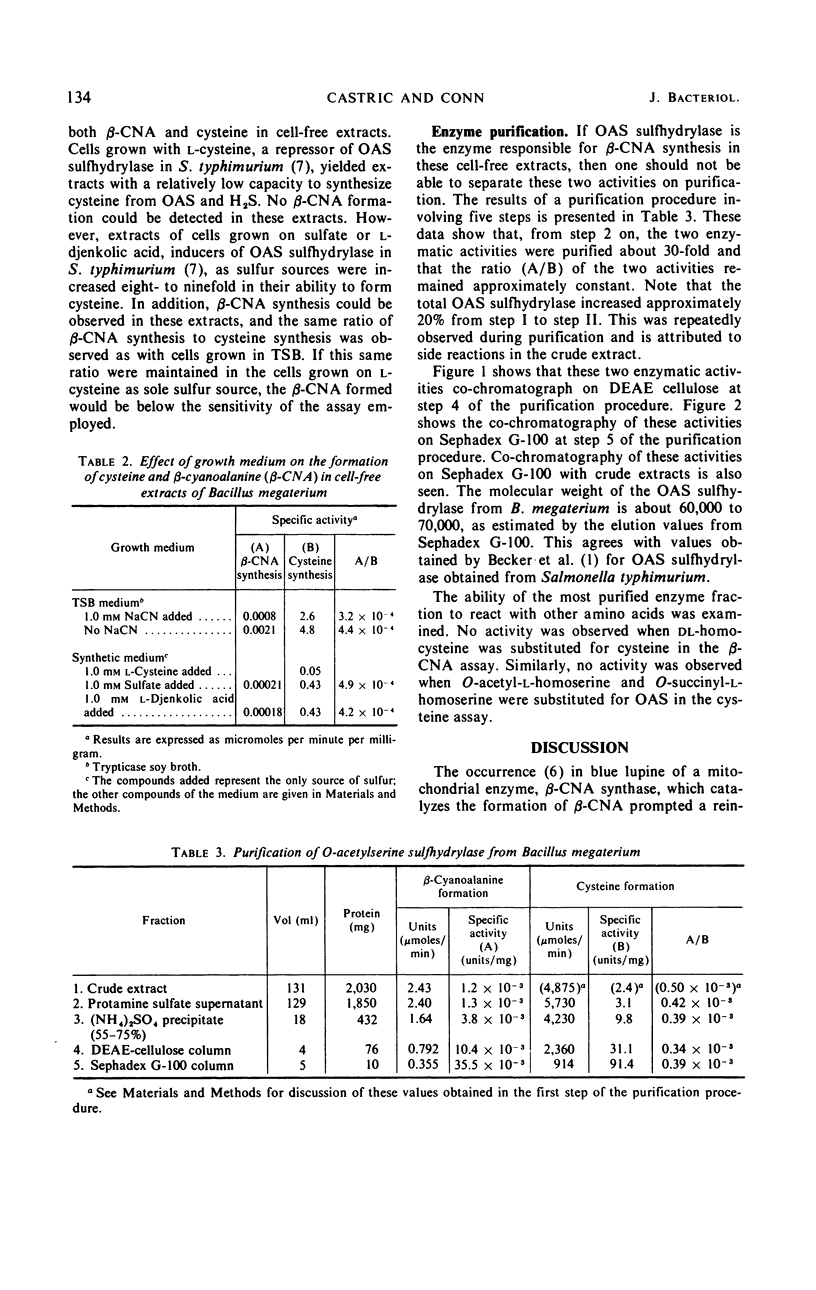
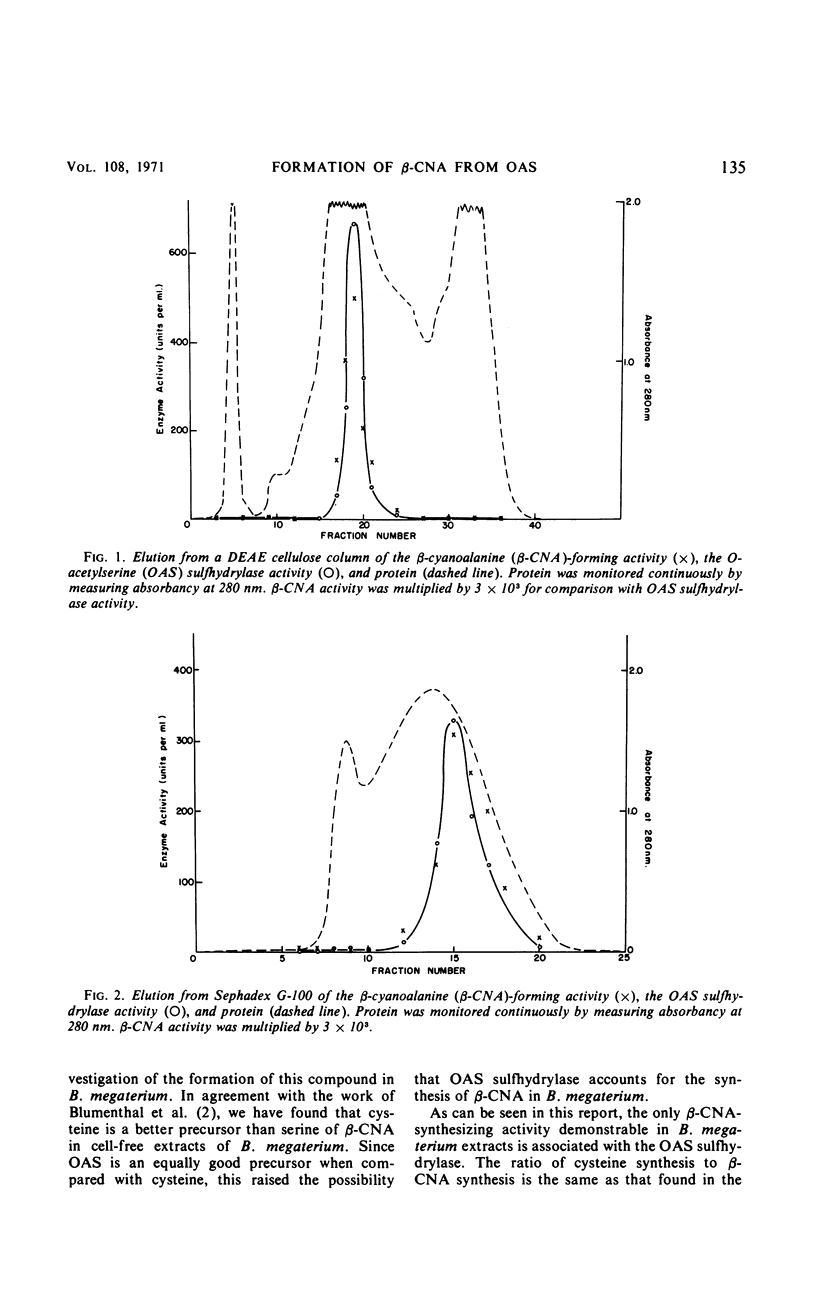

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. A., Kredich N. M., Tomkins G. M. The purification and characterization of O-acetylserine sulfhydrylase-A from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2418–2427. [PubMed] [Google Scholar]
- Blumenthal S. G., Hendrickson H. R., Abrol Y. P., Conn E. E. Cyanide metabolism in higher plants. 3. The biosynthesis of beta-cyanolanine. J Biol Chem. 1968 Oct 25;243(20):5302–5307. [PubMed] [Google Scholar]
- Brysk M. M., Corpe W. A., Hankes L. V. Beta-cyanoalanine formation by Chromobacterium violaceum. J Bacteriol. 1969 Jan;97(1):322–327. doi: 10.1128/jb.97.1.322-327.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castric P. A., Strobel G. A. Cyanide metabolism by Bacillus megaterium. J Biol Chem. 1969 Aug 10;244(15):4089–4094. [PubMed] [Google Scholar]
- Dunnill P. M., Fowden L. Enzymatic formation of beta-cyanoalanine from cyanide by Escherichia coli extracts. Nature. 1965 Dec 18;208(5016):1206–1207. doi: 10.1038/2081206a0. [DOI] [PubMed] [Google Scholar]
- Hendrickson H. R., Conn E. E. Cyanide metabolism in higher plants. IV. Purification and properties of the beta-cyanolanine synthase of blue lupine. J Biol Chem. 1969 May 25;244(10):2632–2640. [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- Mudd S. H., Finkelstein J. D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965 Nov;240(11):4382–4392. [PubMed] [Google Scholar]
- Ressler C., Giza Y. H., Nigam S. N. Beta-cyanoalanine, product of cyanide fixation and intermediate in asparagine biosynthesis in certain species of Lathyrus and Vicia. J Am Chem Soc. 1969 May 7;91(10):2766–2775. doi: 10.1021/ja01038a059. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Zschoche W. C. Asparagine biosynthesis by cotton roots. Carbon dioxide fixation and cyanide incorporation. Plant Physiol. 1970 Apr;45(4):429–434. doi: 10.1104/pp.45.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]



