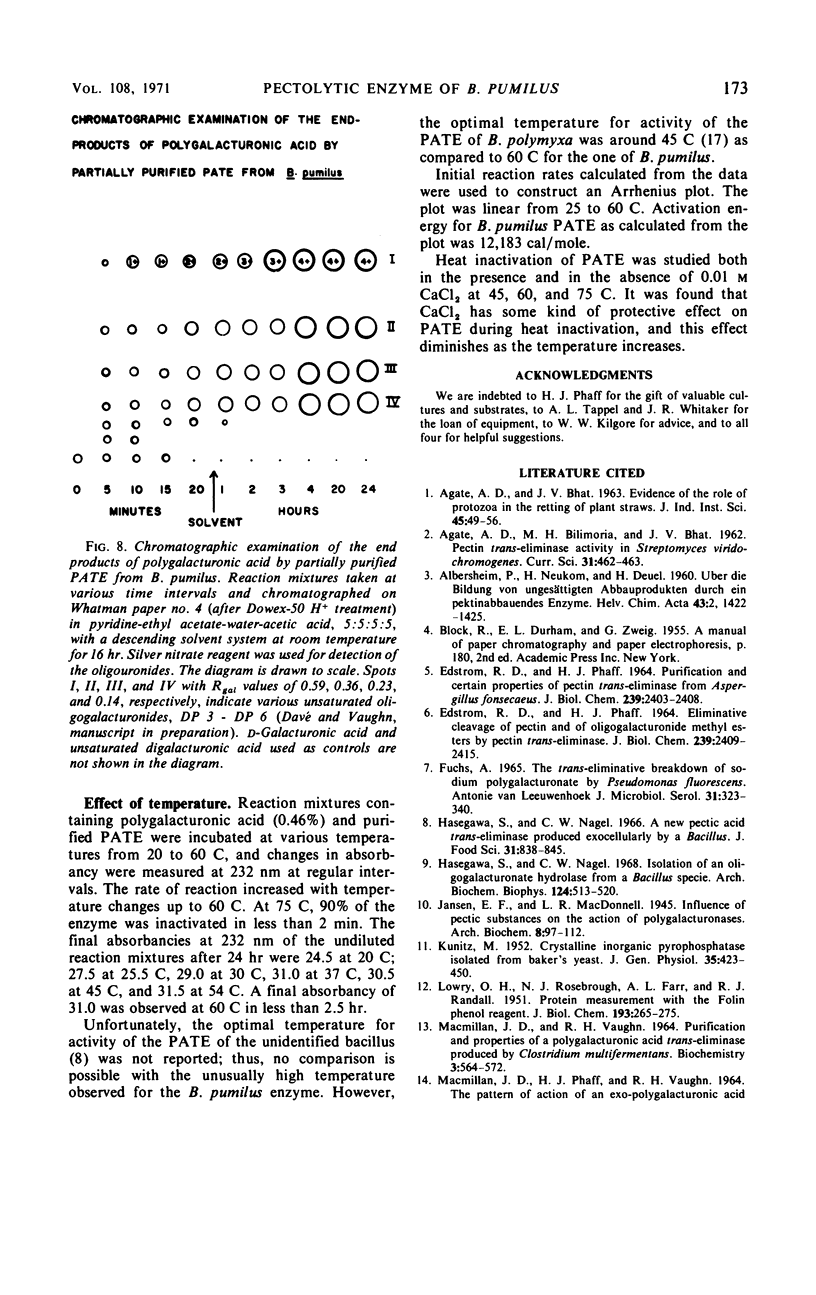
Abstract
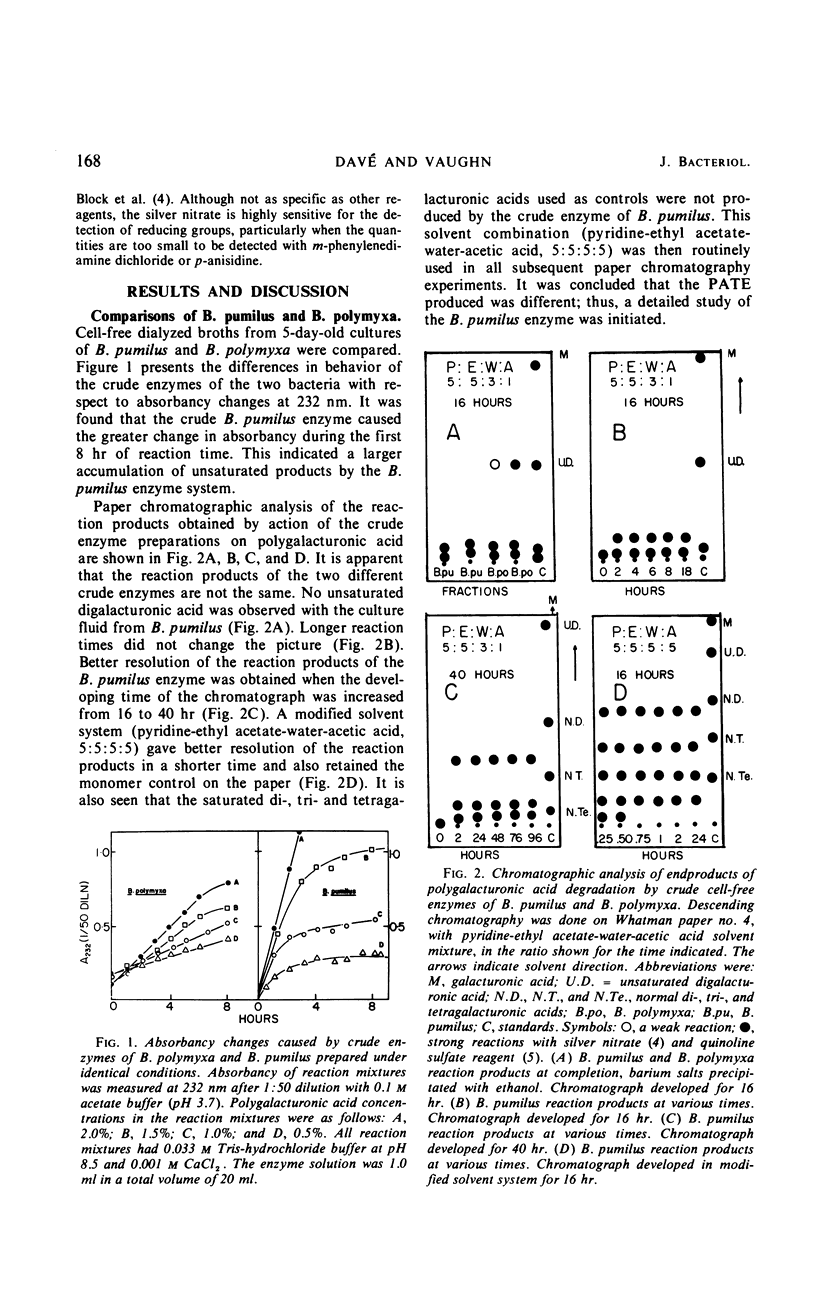
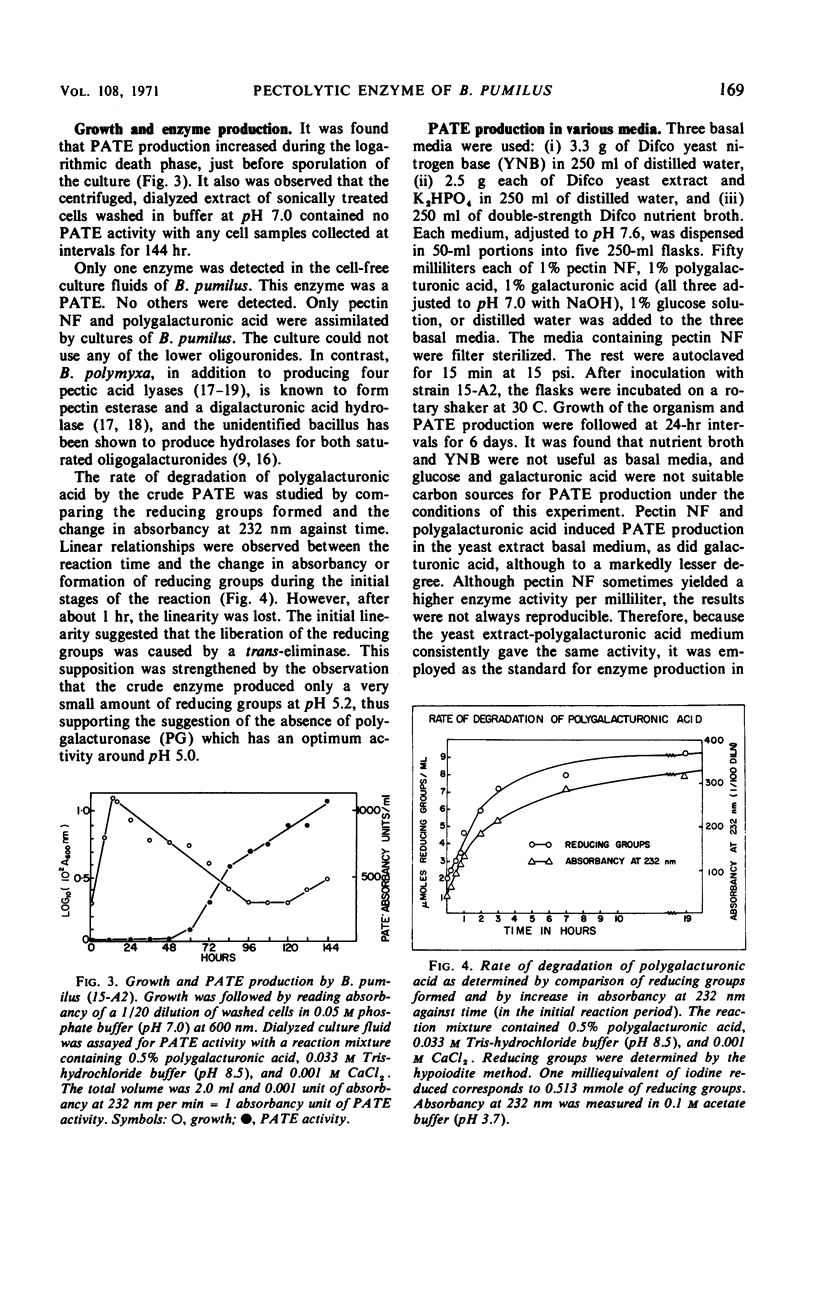
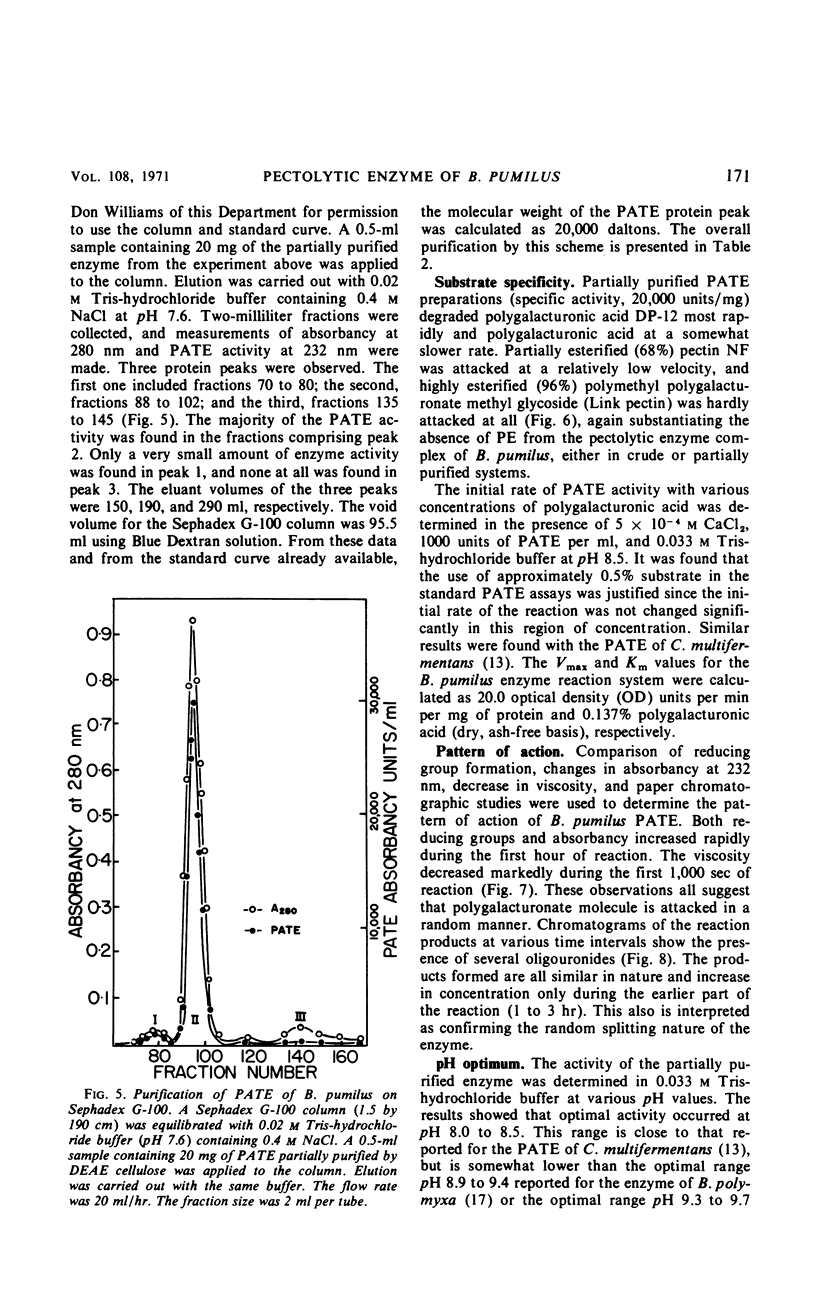
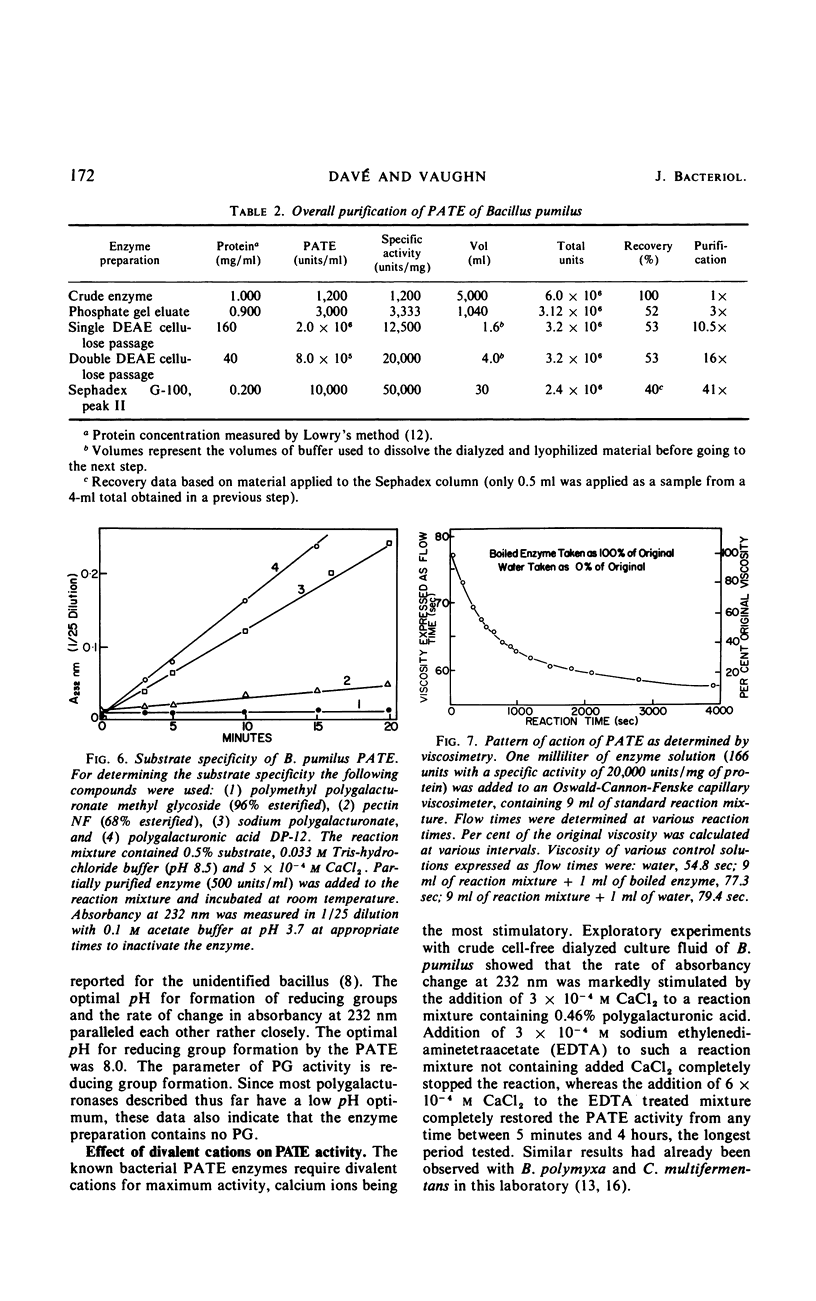
A strain of Bacillus pumilus produced an extracellular pectic enzyme with polygalacturonic acid as the substrate. This enzyme, with optimal activity at pH 8.0 to 8.5, produced reaction products that strongly absorbed light at 232 nm, indicating the presence of a pectic acid trans-eliminase (PATE). Neither pectin esterase nor polygalacturonase was detected in the cell-free culture fluid. Chromatographic examination of the end products revealed the presence of large quantities of unsaturated oligouronides unlike those found with B. polymyxa. It was found that the PATE was produced extracellularly during the negative logarithmic death phase of the organism. The filtrate from sonically treated cells did not show any activity for PATE or hydrolases for lower oligogalacturonides at any time during the growth cycle. The enzyme was inducible. Pectin, National Formulary (NF) was the best inducer, followed by polygalacturonic acid and galacturonic acid. Enzyme activity was markedly stimulated by calcium and other divalent ions. Copper and cobalt ions were inhibitory. The partially purified enzyme showed no significant activity on pectin containing a high methoxyl content (96% esterified). However, pectin NF with a lower methoxyl content (68% esterified) was attacked to a degree by the partially purified and crude enzyme preparations. The initial rate of PATE activity increased up to 60 C, about 16-fold higher than that observed at room temperature. The activation energy was calculated as 12,183 cal/mole. A protective action of calcium chloride against heat inactivation of the PATE was observed. Degradation of polygalacturonic acid by this enzyme produced several unsaturated oligouronides soon after its addition to the substrate. The major endproduct was thought to be different from that of other known PATE enzymes. Paper chromatographic studies and viscosity measurements disclosed the random cleaving nature of the enzyme an endo-PATE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EDSTROM R. D., PHAFF H. J. ELIMINATIVE CLEAVAGE OF PECTIN AND OF OLIGOGALACTURONIDE METHYL ESTERS BY PECTIN TRANS-ELIMINASE. J Biol Chem. 1964 Aug;239:2409–2415. [PubMed] [Google Scholar]
- EDSTROM R. D., PHAFF H. J. PURIFICATION AND CERTAIN PROPERTIES OF PECTIN TRANS-ELIMINASE FROM ASPERGILLUS FONSECAEUS. J Biol Chem. 1964 Aug;239:2403–2408. [PubMed] [Google Scholar]
- Fuchs A. The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie Van Leeuwenhoek. 1965;31(3):323–340. doi: 10.1007/BF02045912. [DOI] [PubMed] [Google Scholar]
- Hasegawa S., Nagel C. W. Isolation of an oligogalacturonate hydrolase from a Bacillus specie. Arch Biochem Biophys. 1968 Mar 20;124(1):513–520. doi: 10.1016/0003-9861(68)90360-3. [DOI] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline inorganic pyrophosphatase isolated from baker's yeast. J Gen Physiol. 1952 Jan;35(3):423–450. doi: 10.1085/jgp.35.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACMILLAN J. D., PHAFF H. J., VAUGHN R. H. THE PATTERN OF ACTION OF AN EXOPOLYGALACTURONIC ACID-TRANS-ELIMINASE FROM CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:572–578. doi: 10.1021/bi00892a017. [DOI] [PubMed] [Google Scholar]
- MACMILLAN J. D., VAUGHN R. H. PURIFICATION AND PROPERTIES OF A POLYGALACTURONIC ACID-TRANS-ELIMINASE PRODUCED BY CLOSTRIDIUM MULTIFERMENTANS. Biochemistry. 1964 Apr;3:564–572. doi: 10.1021/bi00892a016. [DOI] [PubMed] [Google Scholar]
- McCOMB E. A., McCREADY R. M. Use of the hydroxamic acid reaction for determining pectinesterase activity. Stain Technol. 1958 May;33(3):129–131. doi: 10.3109/10520295809111836. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The characteristics of a polygalacturonase produced by Bacillus polymyxa. Arch Biochem Biophys. 1961 May;93:344–352. doi: 10.1016/0003-9861(61)90277-6. [DOI] [PubMed] [Google Scholar]
- NAGEL C. W., VAUGHN R. H. The degradation of oligogalacturonides by the polygalacturonase of Bacillus polymyxa. Arch Biochem Biophys. 1961 Aug;94:328–332. doi: 10.1016/0003-9861(61)90047-9. [DOI] [PubMed] [Google Scholar]
- Nagel C. W., Wilson T. M. Pectic acid lyases of Bacillus polymyxa. Appl Microbiol. 1970 Sep;20(3):374–383. doi: 10.1128/am.20.3.374-383.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Pectic enzymes of pseudomonas marginalis. Phytopathology. 1966 Dec;56(12):1414–1415. [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonase of Erwinia carotovora. J Biol Chem. 1966 Nov 25;241(22):5298–5306. [PubMed] [Google Scholar]
- Nasuno S., Starr M. P. Polygalacturonic acid trans-eliminase of Xanthomonas campestris. Biochem J. 1967 Jul;104(1):178–185. doi: 10.1042/bj1040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. I. Enzymatic formation of 4-deoxy-L-threo-5-hexoseulose uronic acid. J Biol Chem. 1963 May;238:1571–1583. [PubMed] [Google Scholar]
- PREISS J., ASHWELL G. Polygalacturonic acid metabolism in bacteria. II. Formation and metabolism of 3-deoxy-D-glycero-2, 5-hexodiulosonic acid. J Biol Chem. 1963 May;238:1577–1583. [PubMed] [Google Scholar]
- STARR M. P., MORAN F. Eliminative split of pectic substances by phytopathogenic soft-rot bacteria. Science. 1962 Mar 16;135(3507):920–921. doi: 10.1126/science.135.3507.920. [DOI] [PubMed] [Google Scholar]
- Vaughn R. H., Jakubczyk T., Macmillan J. D., Higgins T. E., Dave B. A., Crampton V. M. Some pink yeasts associated with softening of olives. Appl Microbiol. 1969 Nov;18(5):771–775. doi: 10.1128/am.18.5.771-775.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


