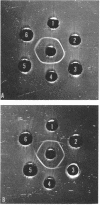
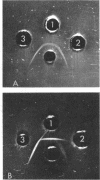
Abstract
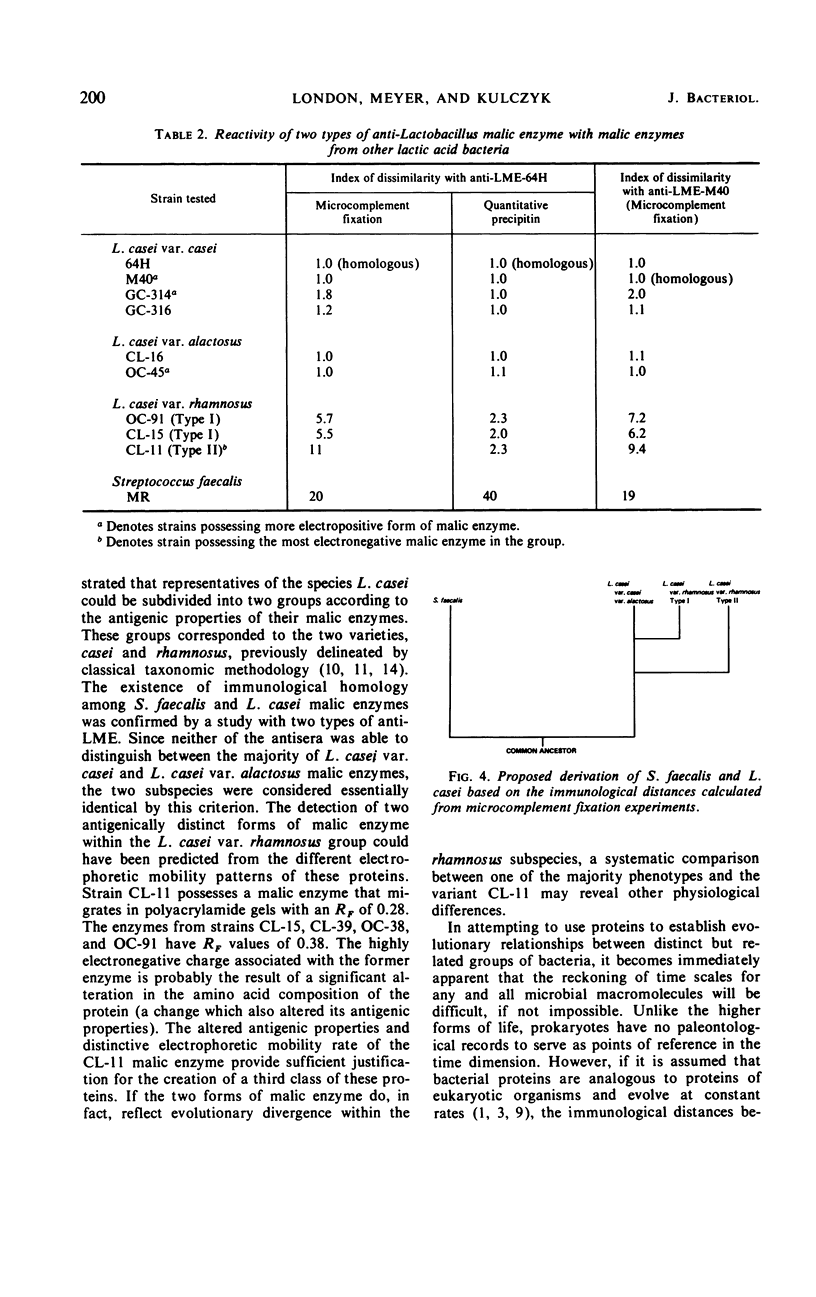
Antisera prepared against two isologous malic enzymes from Lactobacillus casei strains 64H and M40 were used to survey and categorize the various malic enzymes found within this diverse species. In addition to detecting three major antigenic variants of malic enzyme within this group, both antisera readily reacted with Streptococcus faecalis malic enzyme. The cross-reactions between the L. casei malic enzyme antisera and the S. faecalis malic enzyme indicated that these iso-functional enzymes found in two apparently diverse groups of organisms were immunologically homologous. A scheme proposing a common ancestry for the two species S. faecalis and L. casei based on the results of quantitative immunological studies is presented.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Thompson E. O., Richardson B. J., Sharman G. B. Amino-acid sequences of kangaroo myoglobin and haemoglobin and the date of marsupial-eutherian divergence. Nature. 1971 Feb 5;229(5284):391–394. doi: 10.1038/229391a0. [DOI] [PubMed] [Google Scholar]
- Gasser F., Gasser C. Immunological relationships among lactic dehydrogenases in the genera Lactobacillus and Leuconostoc. J Bacteriol. 1971 Apr;106(1):113–125. doi: 10.1128/jb.106.1.113-125.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J. L., Jukes T. H. Non-Darwinian evolution. Science. 1969 May 16;164(3881):788–798. doi: 10.1126/science.164.3881.788. [DOI] [PubMed] [Google Scholar]
- London J., Meyer E. Y., Kulczyk S. Comparative biochemical and immunological study of malic enzyme from two species of lactic acid bacteria: evolutionary implications. J Bacteriol. 1971 Apr;106(1):126–137. doi: 10.1128/jb.106.1.126-137.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus. II. Evidence for allosteric inhibition of an inducible malate dehydrogenase (decarboxylating) by ATP and glycolytic intermediate products. Biochim Biophys Acta. 1969 Apr 22;178(2):205–212. doi: 10.1016/0005-2744(69)90390-8. [DOI] [PubMed] [Google Scholar]
- London J., Meyer E. Y. Malate utilization by a group D Streptococcus: physiological properties and purification of an inducible malic enzyme. J Bacteriol. 1969 May;98(2):705–711. doi: 10.1128/jb.98.2.705-711.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Meyer E., Kulczyk S. Allosteric control of a Lactobacillus malate dehydrogenase (decarboxylating) by two glycolytic intermediate products. Biochim Biophys Acta. 1970 Sep 16;212(3):512–514. doi: 10.1016/0005-2744(70)90260-3. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan C., Margoliash E. Comparative aspects of primary structures of proteins. Annu Rev Biochem. 1968;37:727–790. doi: 10.1146/annurev.bi.37.070168.003455. [DOI] [PubMed] [Google Scholar]
- ORLAND F. J. A correlation of antigenic characteristics among certain bacteria of the Lactobacillus group. J Infect Dis. 1950 Jan-Feb;86(1):63–80. doi: 10.1093/infdis/86.1.63. [DOI] [PubMed] [Google Scholar]
- PERRIN D. Immunological studies with genetically altered beta-galactosidases. Ann N Y Acad Sci. 1963 May 8;103:1058–1066. doi: 10.1111/j.1749-6632.1963.tb53757.x. [DOI] [PubMed] [Google Scholar]
- ROGOSA M., WISEMAN R. F., MITCHELL J. A., DISRAELY M. N., BEAMAN A. J. Species differentiation of oral lactobacilli from man including description of Lactobacillus salivarius nov spec and lactobacillus Cellobiosus nov spec. J Bacteriol. 1953 Jun;65(6):681–699. doi: 10.1128/jb.65.6.681-699.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Wachter D., Gasser C., Wilson A. C. Comparative immunological studies of two Pseudomonas enzymes. J Bacteriol. 1970 May;102(2):351–362. doi: 10.1128/jb.102.2.351-362.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Whiteside T. L., De Siervo A. J., Salton M. R. Use of antibody to membrane adenosine triphosphatase in the study of bacterial relatioships. J Bacteriol. 1971 Mar;105(3):957–967. doi: 10.1128/jb.105.3.957-967.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]