Abstract
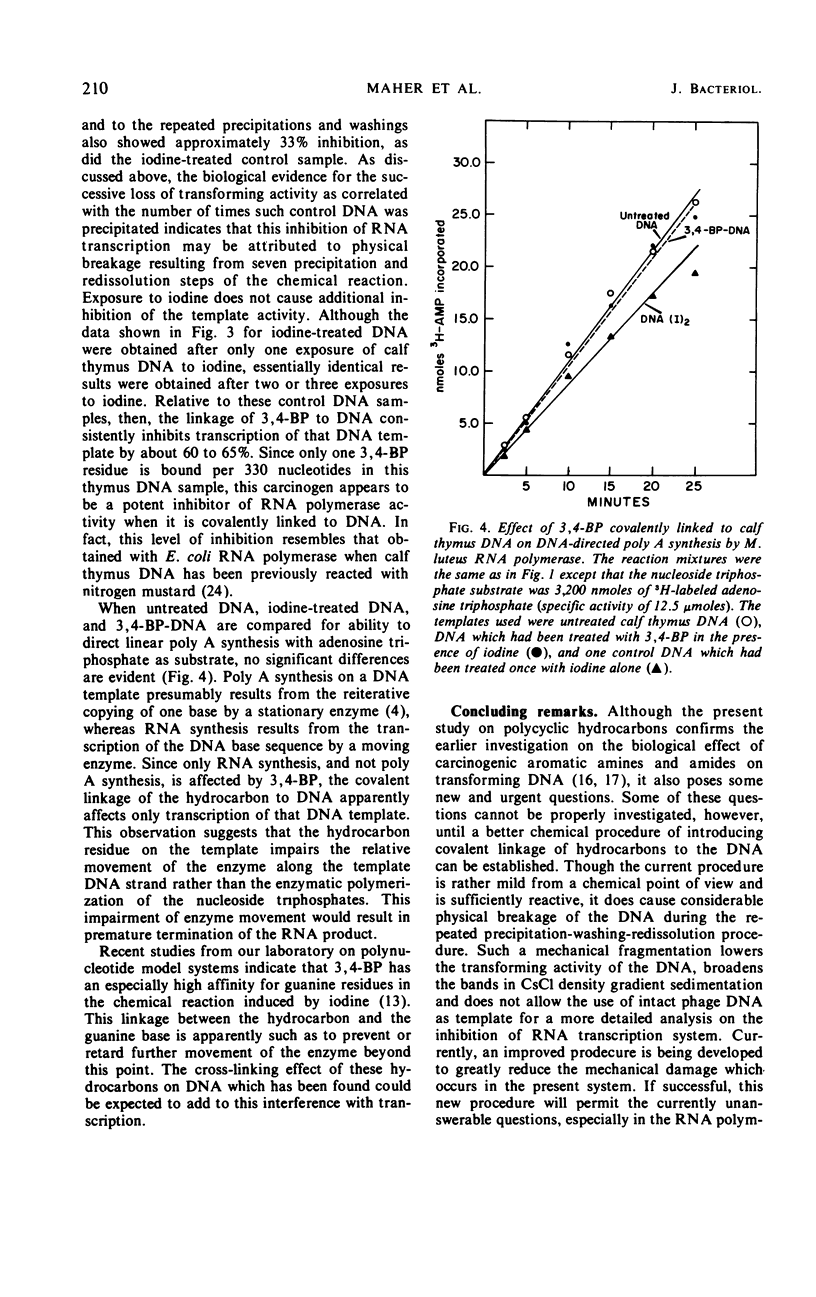
In a solvent system of 10−2m phosphate buffer (pH 6.8)-ethanol (2:1, v/v) and in an iodine-induced reaction, the polycyclic hydrocarbons [3H]3,4-benzpyrene and [3H]3,4-BP/[3H]9, 10-dimethyl-1,2-benzanthracene (DMBA) can be covalently linked to deoxyribonucleic acid (DNA) at room temperature. By stepwise addition of the hydrocarbon and repeating the reaction two to three times after isolating the hydrocarbon DNA adduct, it was possible to introduce as many as one covalently bound hydrocarbon molecule per 100 nucleotide bases. When 3,4-BP and DMBA were linked in this way to biologically active transforming DNA of Bacillus subtilis, they caused (i) reduction of the transforming activity of the DNA accompanied by (ii) significant increases in the frequency of forward mutations. The majority of these hydrocarbon-induced mutations were not able to revert spontaneously. These samples of DNA covalently linked with hydrocarbons showed much lower levels of survival of biological activity when assayed in recipient strains (hcr−) which are known to be deficient in the enzymes required for repair of ultraviolet light-induced damage to DNA. 3,4-BP covalently linked to calf thymus DNA at a level of approximately one hydrocarbon molecule per 330 bases was shown to cause up to 80% inhibition of the in vitro transcription of the DNA by highly purified ribonucleic acid polymerase prepared from Micrococcus luteus under the experimental condition of template saturation. The presence of 3,4-BP and DMBA molecules covalently bound to B. subtilis DNA samples was also found to prevent complete denaturation of the bihelical structure of certain DNA molecules and thus appears to effect a cross-link in these DNA molecules.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKER E. F., Jr, ZIMMERMAN B. K., GEIDUSCHEK E. P. STRUCTURE AND FUNCTION OF CROSS-LINKED DNA. I. REVERSIBLE DENATURATION AND BACILLUS SUBTILIS TRANSFORMATION. J Mol Biol. 1964 Mar;8:377–391. doi: 10.1016/s0022-2836(64)80202-3. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. REACTION OF SOME MUTAGENIC AND CARCINOGENIC COMPOUNDS WITH NUCLEIC ACIDS. J Cell Physiol. 1964 Oct;64:SUPPL 1–1:127. [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett T. H., Heidelberger C., Dove W. F. Determination of the mutagenic activity to bacteriophage T4 of carcinogenic and noncarcinogenic compounds. Mol Pharmacol. 1970 Nov;6(6):667–679. [PubMed] [Google Scholar]
- DiPaolo J. A., Donovan P., Nelson R. Quantitative studies of in vitro transformation by chemical carcinogens. J Natl Cancer Inst. 1969 May;42(5):867–874. [PubMed] [Google Scholar]
- Dipple A., Lawley P. D., Brookes P. Theory of tumour initiation by chemical carcinogens: dependence of activity on structure of ultimate carcinogen. Eur J Cancer. 1968 Oct;4(5):493–506. doi: 10.1016/0014-2964(68)90005-4. [DOI] [PubMed] [Google Scholar]
- Duncan M., Brookes P., Dipple A. Metabolism and binding to cellular macromolecules of a series of hydrocarbons by mouse embryo cells in culture. Int J Cancer. 1969 Nov 15;4(6):813–819. doi: 10.1002/ijc.2910040610. [DOI] [PubMed] [Google Scholar]
- FOX C. F., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. II. PROPERTIES OF THE DEOXYRIBONUCLEIC ACID-PRIMED REACTION WITH MICROCOCCUS LYSODEIKTICUS RIBONUCLEIC ACID POLYMERASE. J Biol Chem. 1964 Jan;239:175–185. [PubMed] [Google Scholar]
- FREESE E., STRACK H. B. Induction of mutations in transforming DNA by hydroxylamine. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1796–1803. doi: 10.1073/pnas.48.10.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Freese E. B. Mutagenic and inactivating DNA alterations. Radiat Res. 1966;(Suppl):97+–97+. [PubMed] [Google Scholar]
- HANAWALT P. C., HAYNES R. H. REPAIR REPLICATION OF DNA IN BACTERIA: IRRELEVANCE OF CHEMICAL NATURE OF BASE DEFECT. Biochem Biophys Res Commun. 1965 May 3;19:462–467. doi: 10.1016/0006-291x(65)90147-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. D., Lesko S. A., Jr, Ts'o P. O. Chemical linkage of polycyclic hydrocarbons to deoxyribonucleic acids and polynucleotides in aqueous solution and in a buffer-ethanol solvent system. Biochemistry. 1970 Jun 23;9(13):2594–2604. doi: 10.1021/bi00815a006. [DOI] [PubMed] [Google Scholar]
- Lesko S. A., Jr, Smith A., Ts'o P. O., Umans R. S. Interaction of nucleic acids. IV. The physical binding of 3,4-benzpyrene to nucleosides, nucleotides, nucleic acids, and nucleoprotein. Biochemistry. 1968 Jan;7(1):434–447. doi: 10.1021/bi00841a056. [DOI] [PubMed] [Google Scholar]
- Lesko S. A., Jr, Ts'o P. O., Umans R. S. Interaction of nucleic acids. V. Chemical linkage of 3,4-benzpyrene to deoxyribonucleic acid in aqueous solution. Biochemistry. 1969 Jun;8(6):2291–2298. doi: 10.1021/bi00834a009. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Miller E. C., Miller J. A., Szybalski W. Mutations and decreases in density of transforming DNA produced by derivatives of the carcinogens 2-acetyl-aminofluorene and N-methyl-4-aminoazobenzene. Mol Pharmacol. 1968 Sep;4(5):411–426. [PubMed] [Google Scholar]
- Maher V. M., Miller J. A., Miller E. C., Summers W. C. Mutations and loss of transforming activity of Bacillus subtilis DNA after reaction with esters of carcinogenic N-hydroxy aromatic amides. Cancer Res. 1970 May;30(5):1473–1480. [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Mechanisms of chemical carcinogenesis: nature of proximate carcinogens and interactions with macromolecules. Pharmacol Rev. 1966 Mar;18(1):805–838. [PubMed] [Google Scholar]
- Miller J. A., Miller E. C. The metabolic activation of carcinogenic aromatic amines and amides. Prog Exp Tumor Res. 1969;11:273–301. doi: 10.1159/000391399. [DOI] [PubMed] [Google Scholar]
- OPARA-KUBINSKA Z., KURYLO-BOROWSKA Z., SZYBALSKI W. Genetic transformation studies. II. Effect of ultraviolet light on the molecular properties of normal and halogenated deoxyribonucleic acid. Biochim Biophys Acta. 1963 Jun 25;72:298–309. doi: 10.1016/0006-3002(63)90245-2. [DOI] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Comparison of ultraviolet sensitivity of Bacillus subtilis bacteriophage SPO2 and its infectious DNA. J Mol Biol. 1965 Nov;14(1):130–142. doi: 10.1016/s0022-2836(65)80235-2. [DOI] [PubMed] [Google Scholar]
- Rapaport S. A., Ts'o P. O. Interaction of nucleic acids. 3. Chemical linkage of the carcinogen 3,4-benzpyrene to DNA induced by x-ray irradiation. Proc Natl Acad Sci U S A. 1966 Feb;55(2):381–387. doi: 10.1073/pnas.55.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter H., Strauss B. Repair of damage induced by a monofunctional alkylating agent in a transformable, ultraviolet-sensitive strain of Bacillus subtilis. J Mol Biol. 1965 Nov;14(1):179–194. doi: 10.1016/s0022-2836(65)80239-x. [DOI] [PubMed] [Google Scholar]
- Ruddon R. W., Johnson J. M. The effects of nitrogen mustard on DNA template activity in purified DNA and RNA polymerase systems. Mol Pharmacol. 1968 May;4(3):258–273. [PubMed] [Google Scholar]
- Straat P. A., Ts'o P. O., Bollum F. J. Ribonucleic acid polymerase from Micrococcus lysodeikticus. I. Studies on single stranded homopolynucleotide templates containing adenine, thymine, and uracil. J Biol Chem. 1968 Oct 10;243(19):5000–5006. [PubMed] [Google Scholar]
- Straat P. A., Ts'o P. O., Bollum F. J. Ribonucleic acid polymerase from Micrococcus lysodeikticus. II. Studies on double stranded homopolynucleotide templates. J Biol Chem. 1969 Jan 25;244(2):391–398. [PubMed] [Google Scholar]
- Straat P. A., Ts'o P. O. Ribonucleic acid polymerase from Micrococcus luteus (Micrococcus lysodeikticus). IV. Effect of rifampicin and oligomers on the homopolymer-directed reaction. Biochemistry. 1970 Feb 17;9(4):926–931. doi: 10.1021/bi00806a031. [DOI] [PubMed] [Google Scholar]
- Straat P. A., Ts'o P. O. Ribonucleic acid polymerase from Micrococcus luteus. 3. The effect of substrate, metal ion, and temperature on the homopolymer-directed reaction. J Biol Chem. 1969 Nov 25;244(22):6263–6269. [PubMed] [Google Scholar]
- TSO P. O., LU P. INTERACTION OF NUCLEIC ACIDS, II. CHEMICAL LINKAGE OF THE CARCINOGEN 3,4-BENZPYRENE TO DNA INDUCED BY PHOTORADIATION. Proc Natl Acad Sci U S A. 1964 Feb;51:272–280. doi: 10.1073/pnas.51.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umans R. S., Lesko S. A., Jr, Ts'o P. O. Chemical linkage of carcinogenic 3,4-benzpyrene to DNA in aqueous solution induced by peroxide and iodine. Nature. 1969 Feb 22;221(5182):763–764. doi: 10.1038/221763a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]



