Abstract
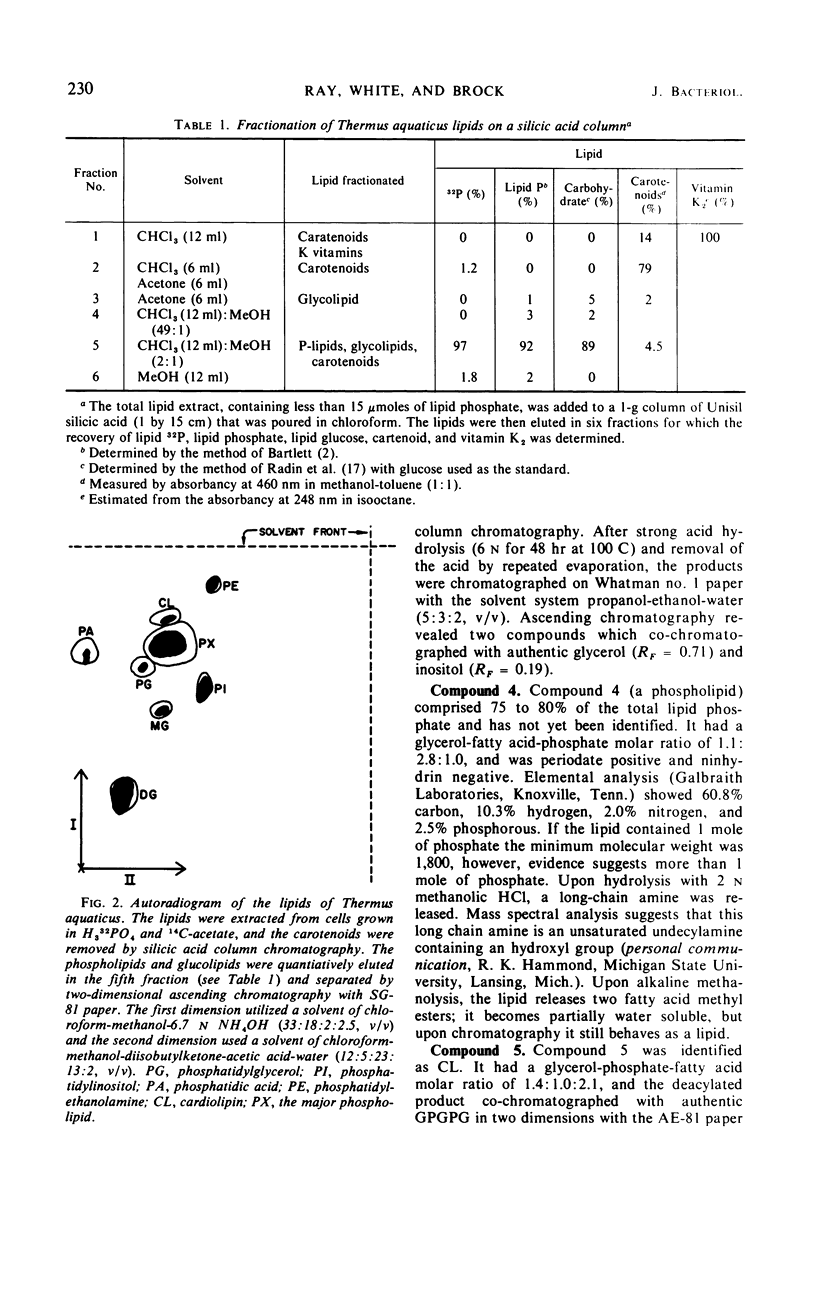
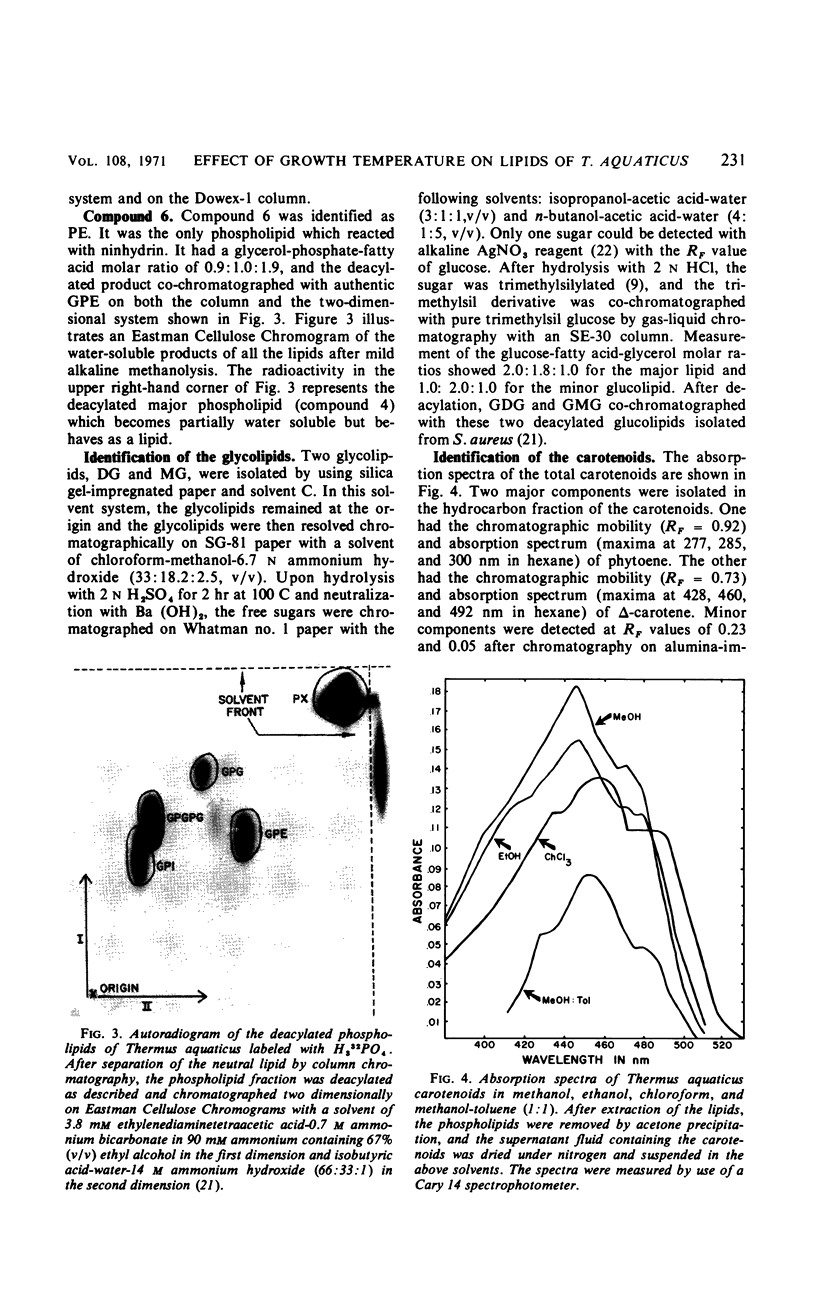
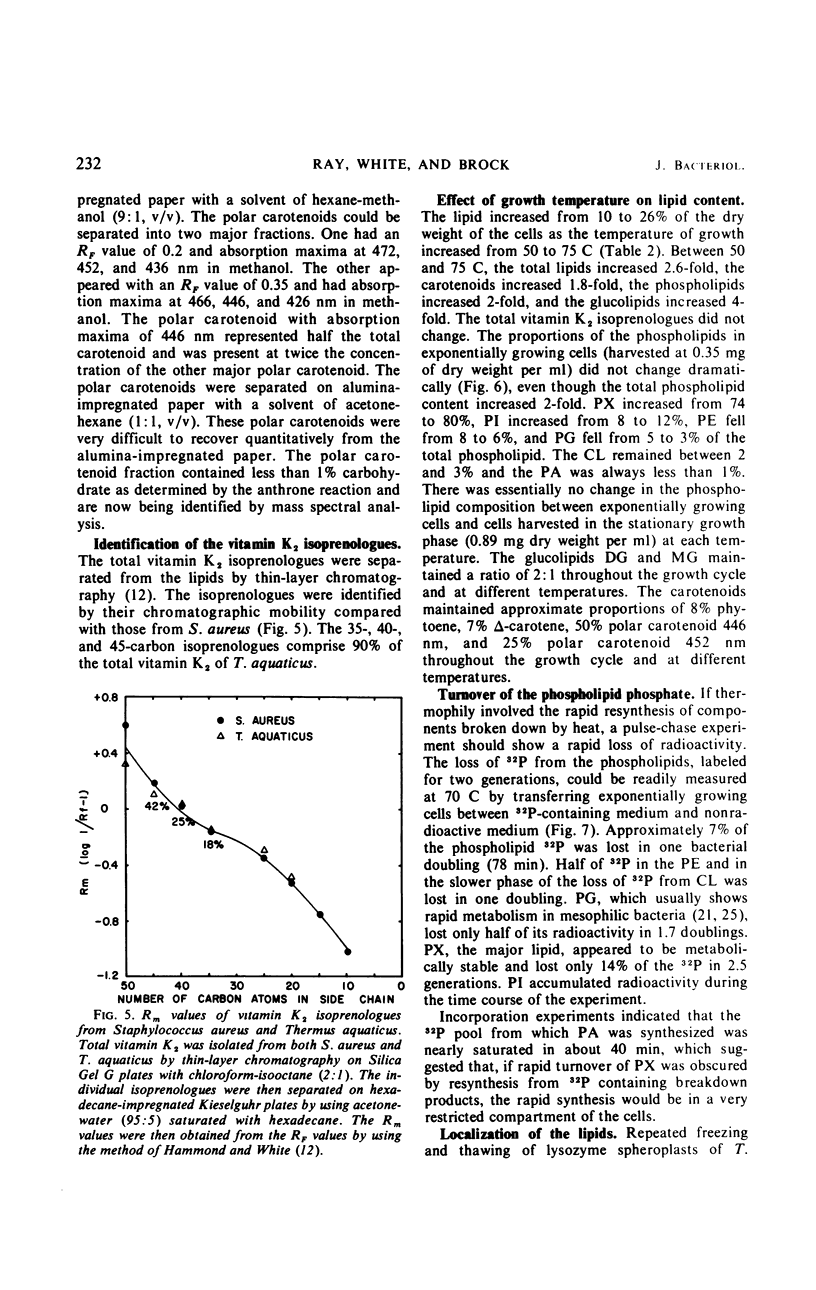
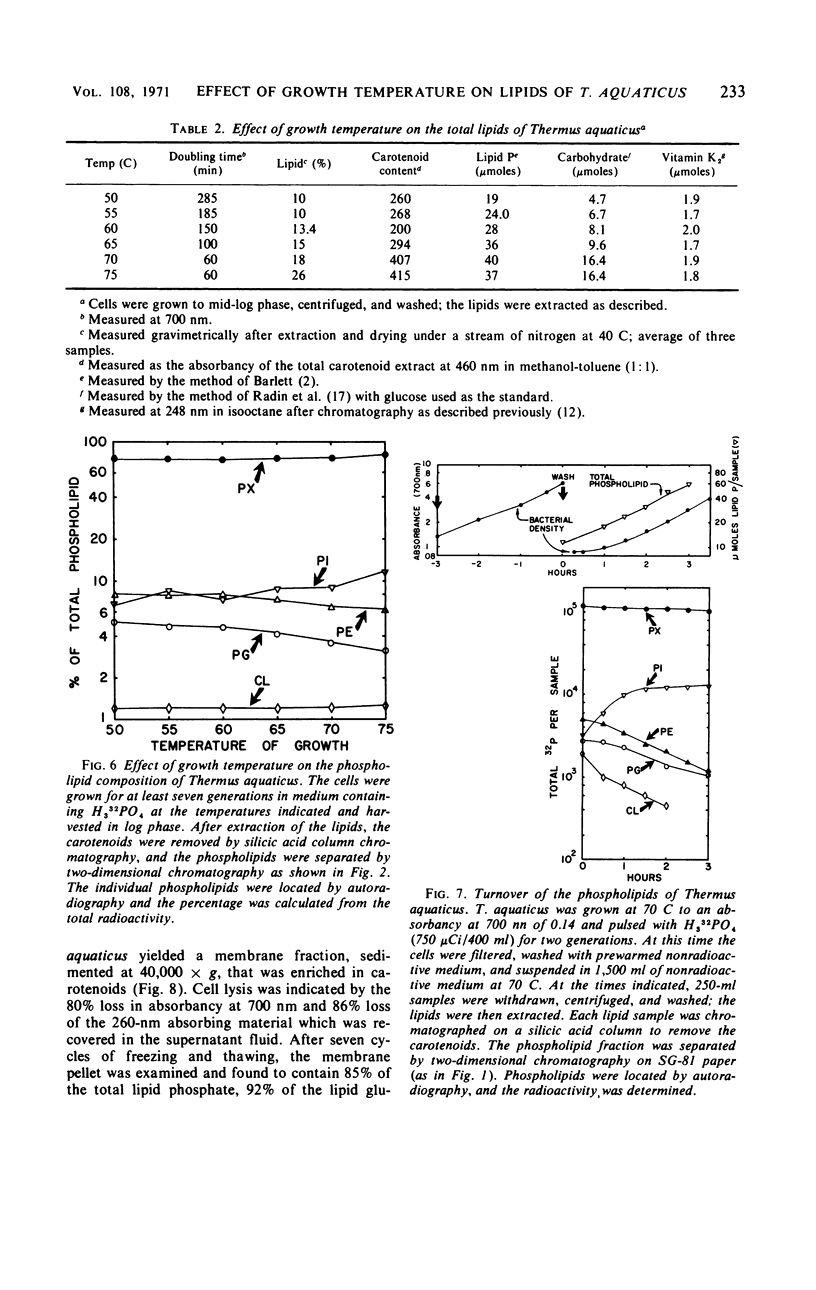
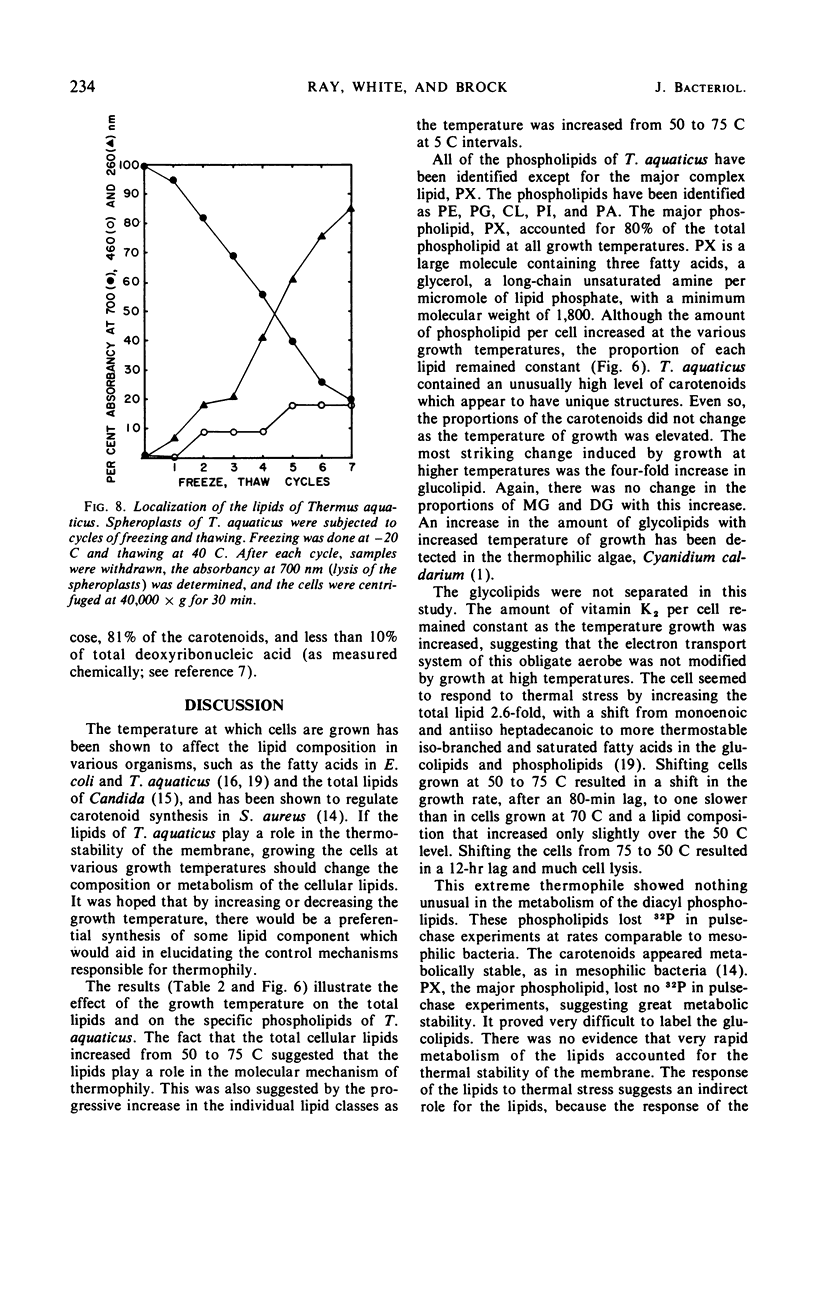
The complex lipids of Thermus aquaticus include phospholipids, glucolipids, carotenoids, and vitamin K2 isoprenologues. The phospholipids account for 30% of the total lipids and have been identified as phosphatidylethanolamine (4%), phosphatidylglycerol (3%), phosphatidylinositol (10%), cardiolipin (3%), and phosphatidic acid (1%). The major phospholipid contained three fatty acids, a long-chain unsaturated amine, and one glycerol per phosphate and accounted for 80% of the lipid phosphate. The carotenoids accounted for 60% of the membrane lipid. The majority of the carotenoids were very polar. Mono- and diglucosyldiglyceride and the 35-, 40-, and 45-carbon vitamin K2 isoprenologues were also identified. All these lipids were localized in the membrane of T. aquaticus. When the growth temperature was increased from 50 to 75 C and measured at 5 C intervals, there was a progressive increase in the total lipid content. The phospholipids increased 2-fold, the carotenoids increased 1.8-fold, and the glucolipids increased 4-fold between cells grown at 50 C and 75 C. The vitamin K2 level did not change. The proportions of the individual lipids within each lipid class remained constant as the temperature of growth was raised. Metabolic studies indicated turnover of the diacyl phospholipids during pulse-chase experiments at rates comparable with mesophilic bacteria. The major phospholipid and the carotenoids did not turn over.
Full text
PDF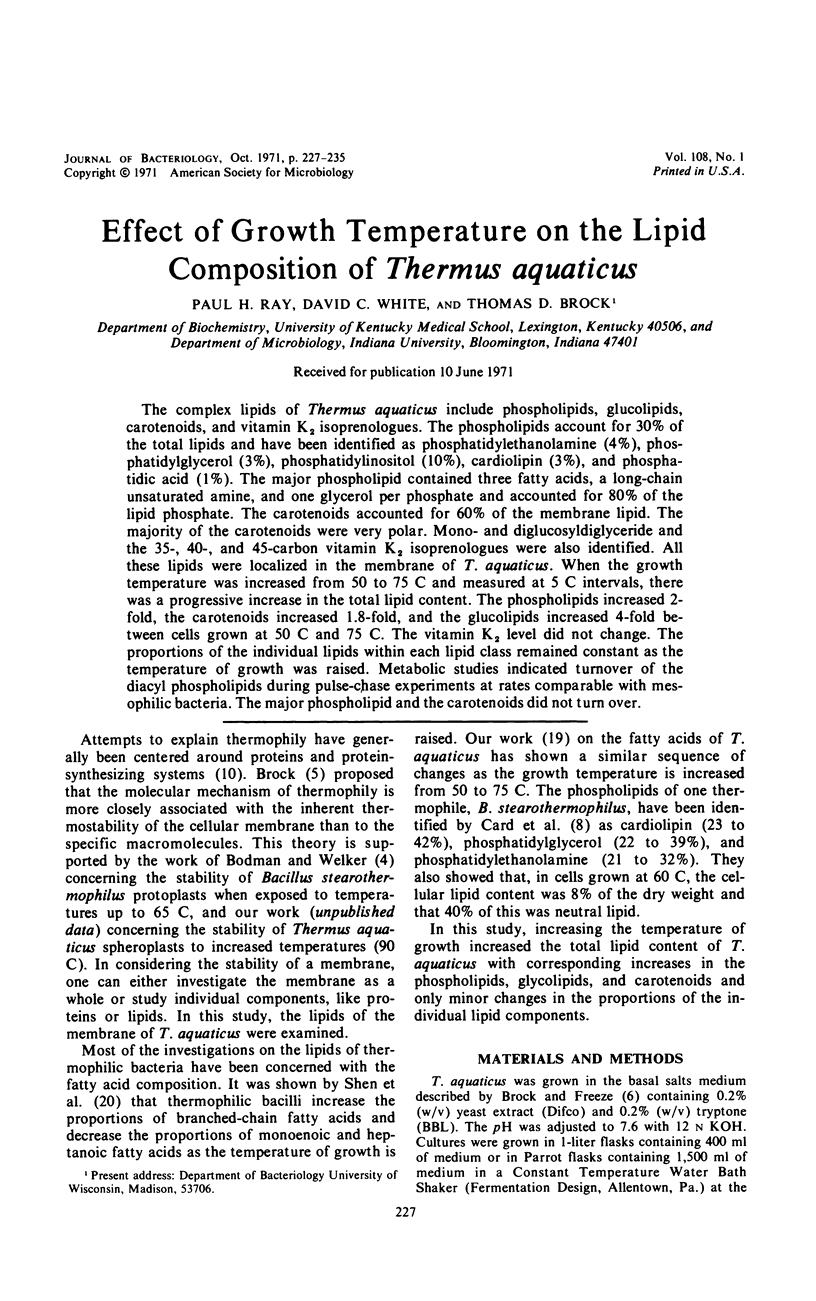


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. L., McMahon V., Seckbach J. Fatty acids in the thermophilic alga, Cyanidium caldarium. Biochem Biophys Res Commun. 1971 Feb 5;42(3):359–365. doi: 10.1016/0006-291x(71)90378-0. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodman H., Welker N. E. Isolation of spheroplast membranes and stability of spheroplasts of Bacillus stearothermophilus. J Bacteriol. 1969 Feb;97(2):924–935. doi: 10.1128/jb.97.2.924-935.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D., Freeze H. Thermus aquaticus gen. n. and sp. n., a nonsporulating extreme thermophile. J Bacteriol. 1969 Apr;98(1):289–297. doi: 10.1128/jb.98.1.289-297.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock T. D. Life at high temperatures. Evolutionary, ecological, and biochemical significance of organisms living in hot springs is discussed. Science. 1967 Nov;158(3804):1012–1019. doi: 10.1126/science.158.3804.1012. [DOI] [PubMed] [Google Scholar]
- Card G. L., Georgi C. E., Militzer W. E. Phospholipids from Bacillus stearothermophilus. J Bacteriol. 1969 Jan;97(1):186–192. doi: 10.1128/jb.97.1.186-192.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Carotenoid formation by Staphylococcus aureus. J Bacteriol. 1970 Jul;103(1):191–198. doi: 10.1128/jb.103.1.191-198.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R. K., White D. C. Separation of vitamin K2 isoprenologues by reversed-phase thin-layer chromatography. J Chromatogr. 1969 Dec 23;45(3):446–452. doi: 10.1016/s0021-9673(01)86242-7. [DOI] [PubMed] [Google Scholar]
- Joyce G. H., Hammond R. K., White D. C. Changes in membrane lipid composition in exponentially growing Staphylococcus aureus during the shift from 37 to 25 C. J Bacteriol. 1970 Oct;104(1):323–330. doi: 10.1128/jb.104.1.323-330.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATES M., BAXTER R. M. Lipid composition of mesophilic and psychrophilic yeasts (Candida species) as influenced by environmental temperature. Can J Biochem Physiol. 1962 Sep;40:1213–1227. [PubMed] [Google Scholar]
- Marr A. G., Ingraham J. L. EFFECT OF TEMPERATURE ON THE COMPOSITION OF FATTY ACIDS IN ESCHERICHIA COLI. J Bacteriol. 1962 Dec;84(6):1260–1267. doi: 10.1128/jb.84.6.1260-1267.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADIN N. S., BROWN J. R., LAVIN F. B. The preparative isolation of cerebrosides. J Biol Chem. 1956 Apr;219(2):977–983. [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- Ray P. H., White D. C., Brock T. D. Effect of temperature on the fatty acid composition of Thermus aquaticus. J Bacteriol. 1971 Apr;106(1):25–30. doi: 10.1128/jb.106.1.25-30.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen P. Y., Coles E., Foote J. L., Stenesh J. Fatty acid distribution in mesophilic and thermophilic strains of the genus Bacillus. J Bacteriol. 1970 Aug;103(2):479–481. doi: 10.1128/jb.103.2.479-481.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Aleem M. I. Phospholipid metabolism in Ferrobacillus ferrooxidans. J Bacteriol. 1969 Jul;99(1):142–150. doi: 10.1128/jb.99.1.142-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- Wuthier R. E. Two-dimensional chromatography on silica gel-loaded paper for the microanalysis of polar lipids. J Lipid Res. 1966 Jul;7(4):544–550. [PubMed] [Google Scholar]