Abstract
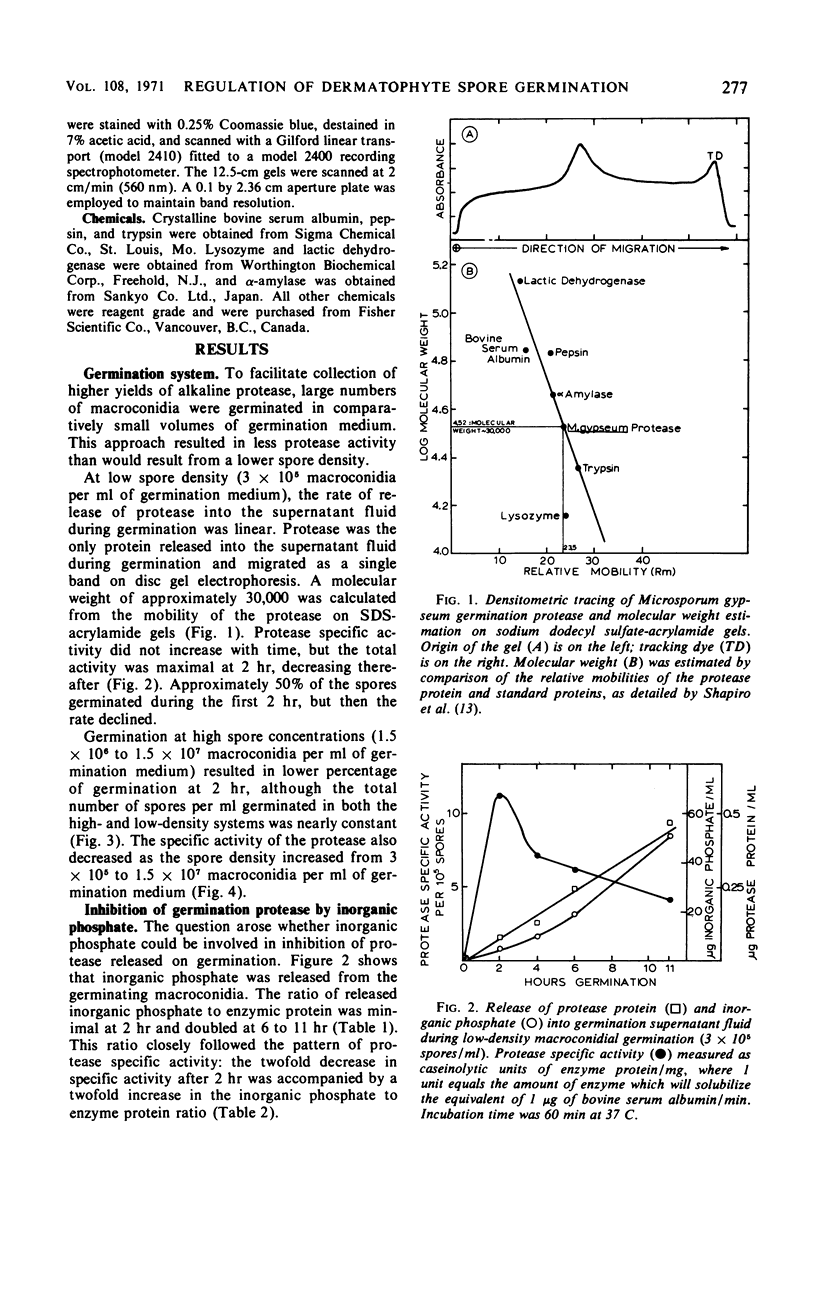
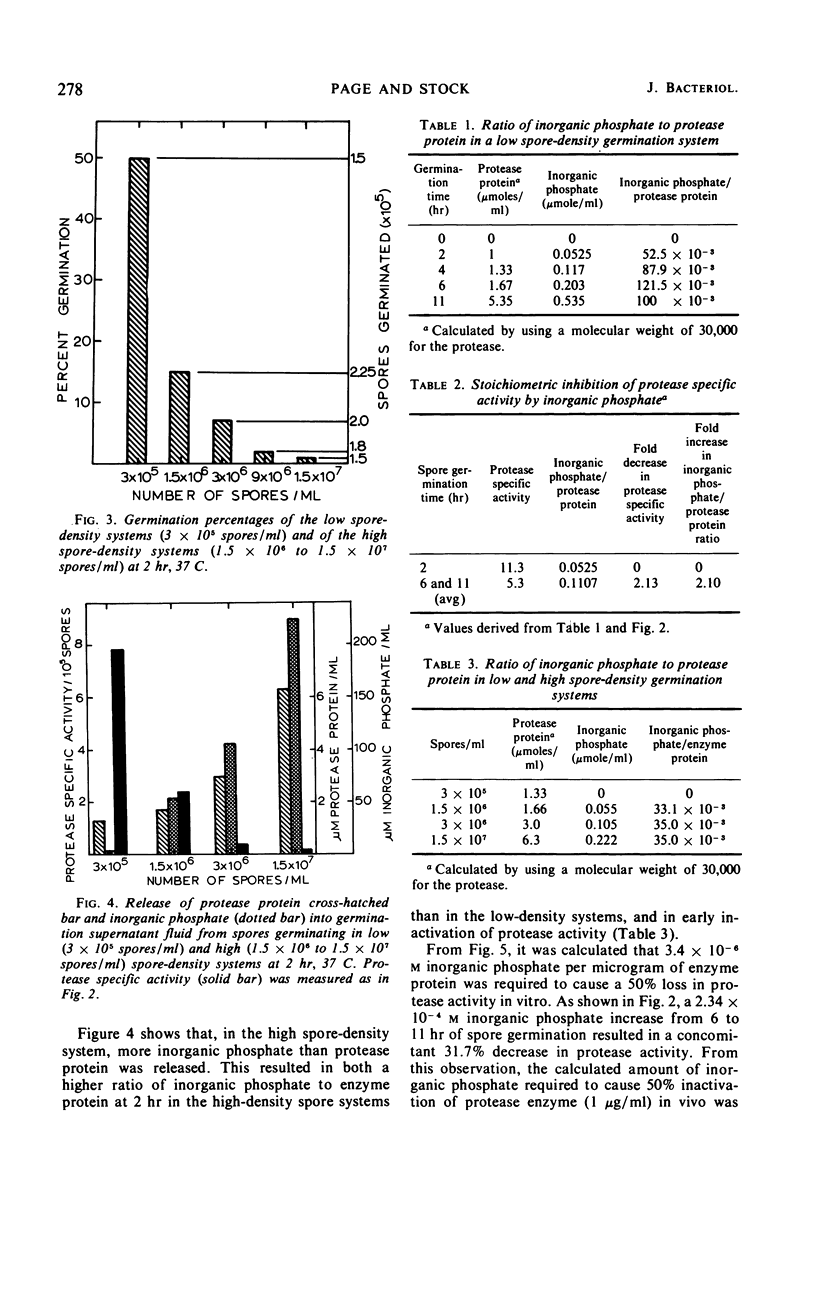
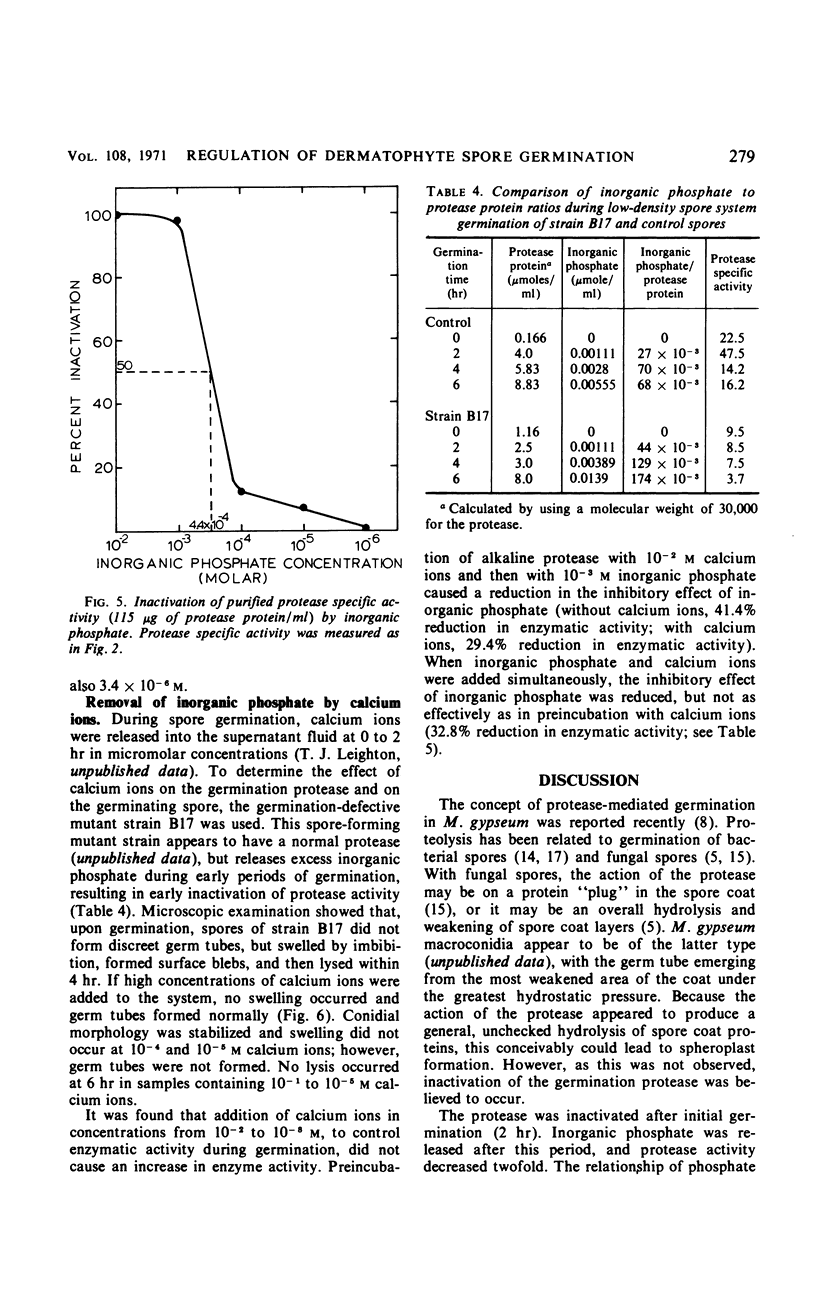
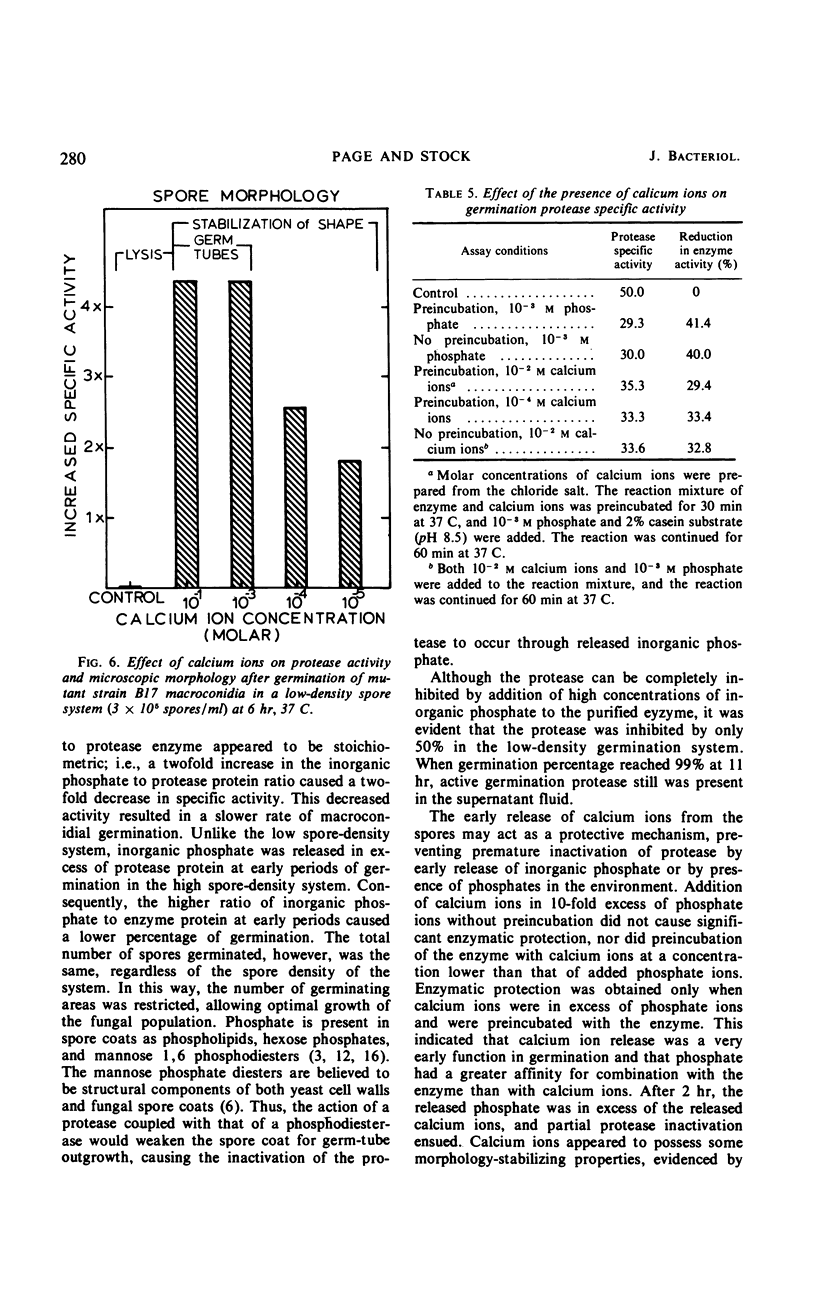
Germination of Microsporum gypseum macroconidia was accompanied by the release of alkaline protease, calcium ions, and inorganic phosphate into the germination fluid. The rate of germination was greatest during the first 2 hr, decreasing thereafter. This decrease in rate was accompanied by a decrease in protease activity, which was caused by an interaction of the enzyme with the inorganic phosphate released from the spores and accumulated in the germination medium after 2 hr. Germination of high spore densities was regulated by the ratio of released phosphate to protease protein, resulting in a constant percentage of germination at both high and low spore densities. A germination-defective mutant strain failed to germinate normally and released excessively high concentrations of phosphate into the germination medium during the initial 2 hr of incubation. Addition of calcium ions to germination mutant macroconidia stabilized spore morphology, prevented protease inactivation, and allowed normal germ-tube outgrowth. The germination of macroconidia appears to be regulated by the release of phosphate ions, which then inhibit the alkaline protease.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HAROLD F. M. Binding of inorganic polyphosphate to the cell wall of Neurospora crassa. Biochim Biophys Acta. 1962 Feb 12;57:59–66. doi: 10.1016/0006-3002(62)91077-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leighton T. J., Stock J. J. Biochemical changes during fungal sporulation and spore germination. I. Phenyl methyl sulfonyl fluoride inhibition of macroconidial germination in Microsporum gypseum. J Bacteriol. 1970 Mar;101(3):931–940. doi: 10.1128/jb.101.3.931-940.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Stock J. J. Heat-induced macroconidia germination in Microsporum gypseum. Appl Microbiol. 1969 Mar;17(3):473–475. doi: 10.1128/am.17.3.473-475.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton T. J., Stock J. J. Isolation and preliminary characterization of developmental mutants from Microsporum gypseum. J Bacteriol. 1970 Nov;104(2):834–838. doi: 10.1128/jb.104.2.834-838.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- Mill P. J. Phosphomannans and other components of flocculent and non-flocculent walls of Saccharomyces cerevisiae. J Gen Microbiol. 1966 Sep;44(3):329–341. doi: 10.1099/00221287-44-3-329. [DOI] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. A cell-wall lytic enzyme associated with spores of Bacillus species. J Gen Microbiol. 1957 Feb;16(1):236–249. doi: 10.1099/00221287-16-1-236. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Sierra G. Germination of bacterial endospores with subtilopeptidases. Can J Microbiol. 1967 May;13(5):489–501. doi: 10.1139/m67-064. [DOI] [PubMed] [Google Scholar]
- Trinci A. P. A kinetic study of the growth of Aspergillus nidulans and other fungi. J Gen Microbiol. 1969 Jul;57(1):11–24. doi: 10.1099/00221287-57-1-11. [DOI] [PubMed] [Google Scholar]


