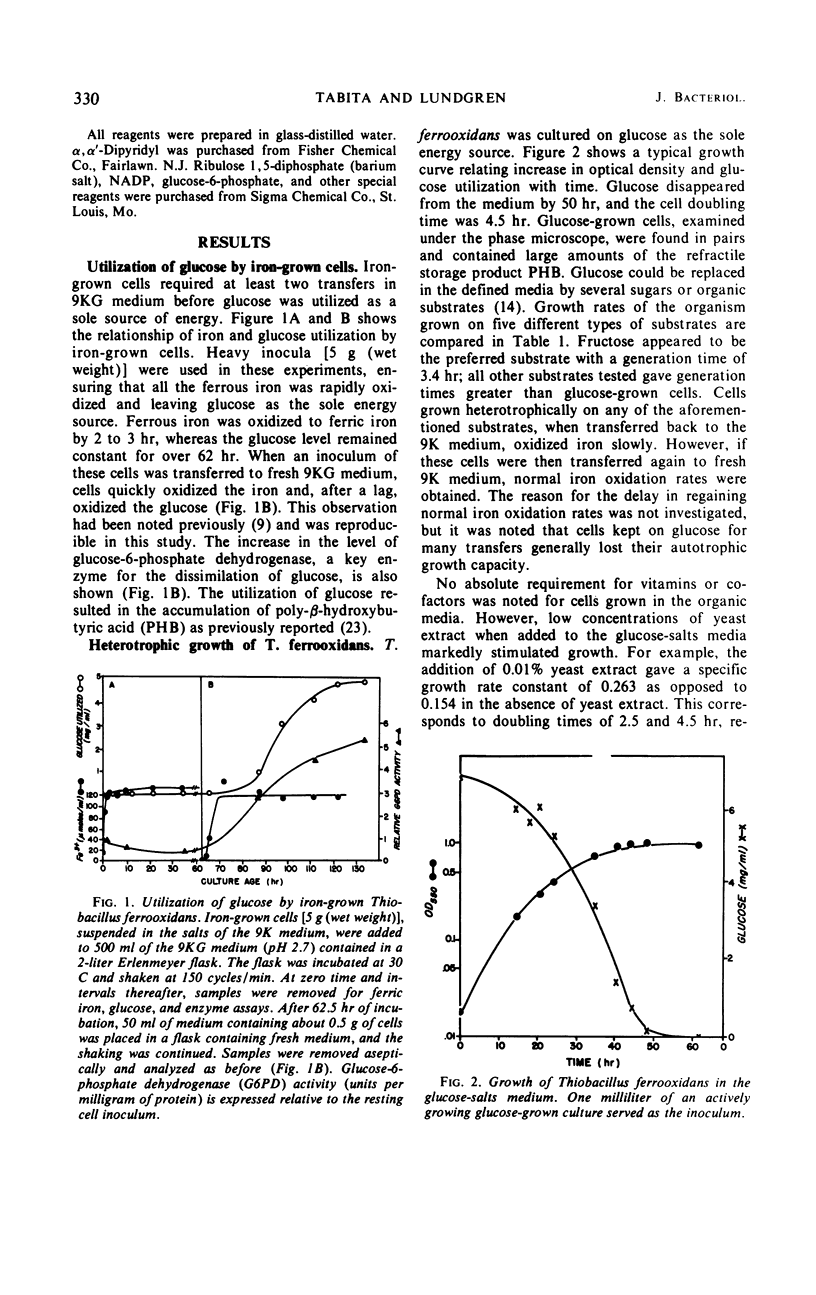
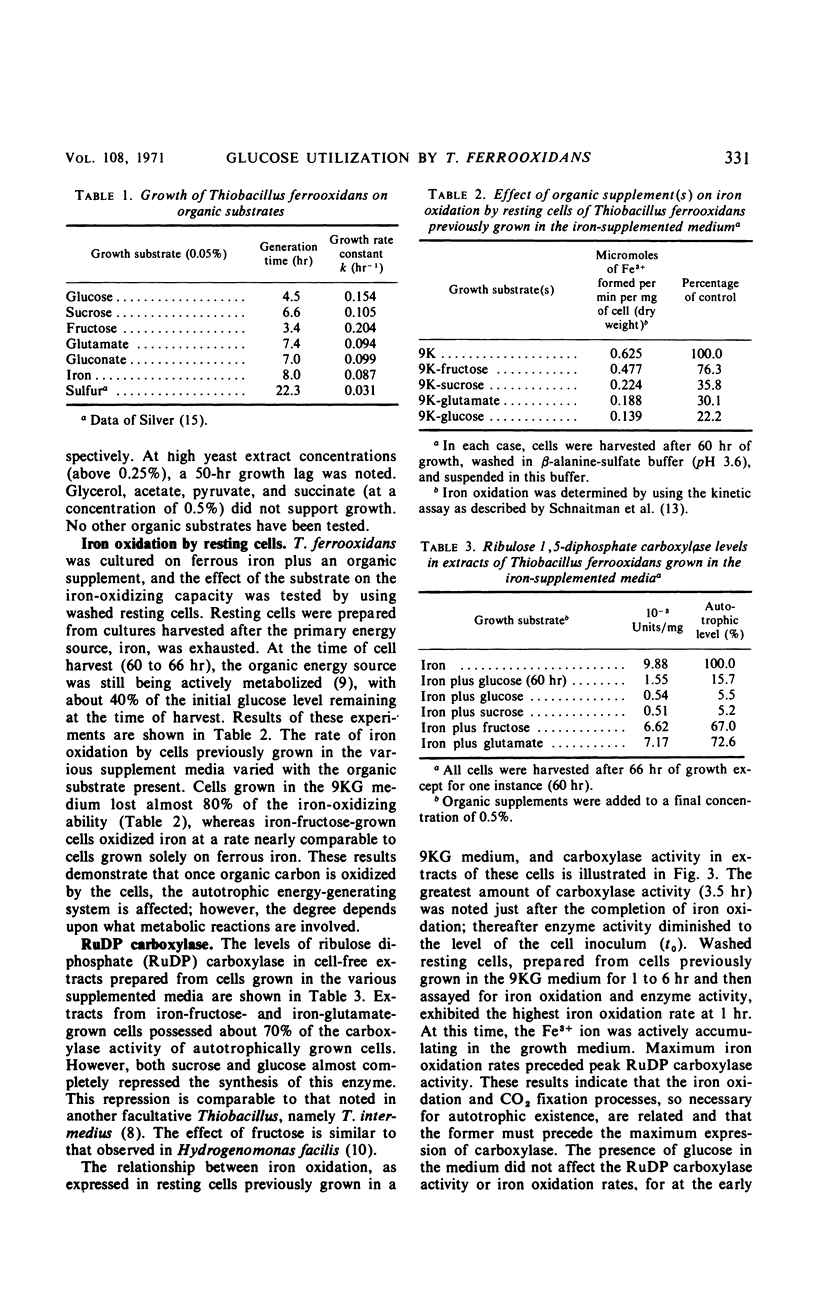
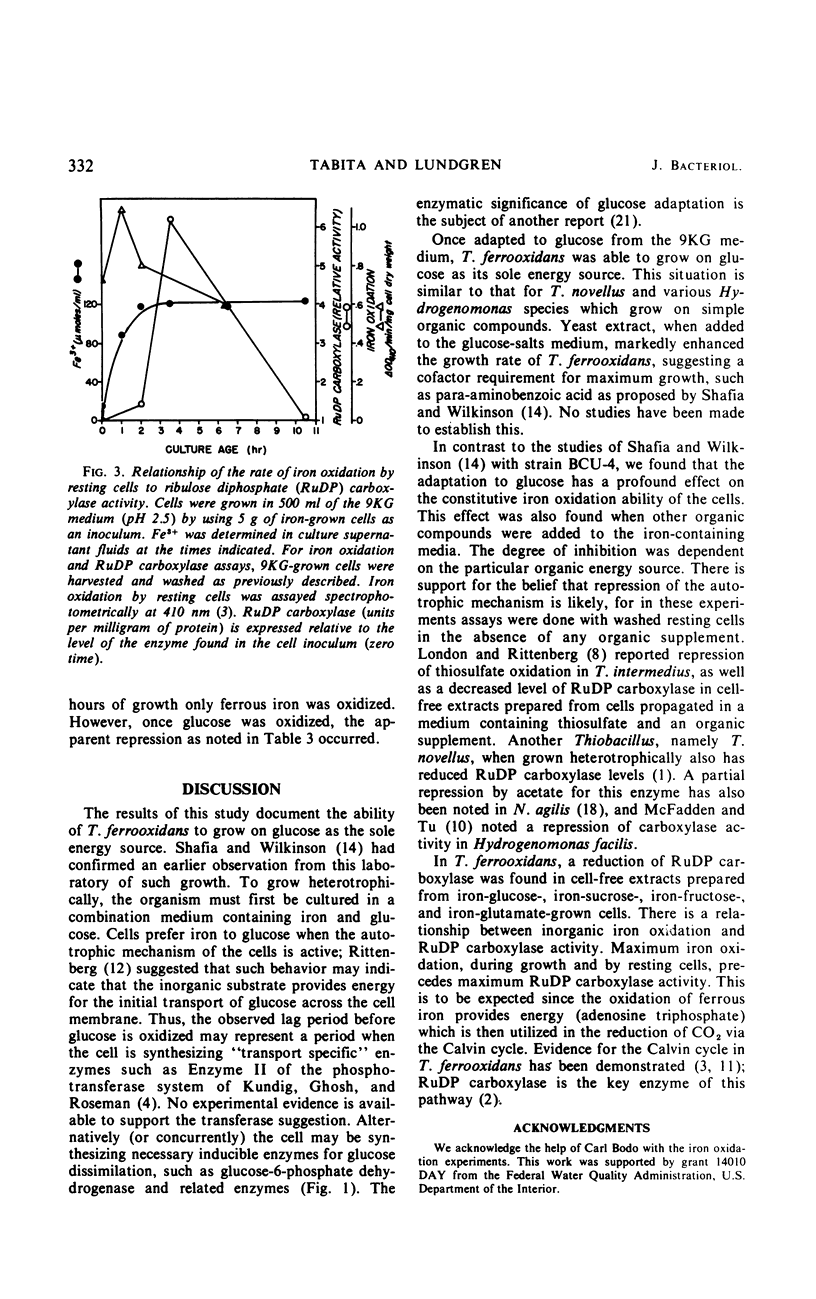
Abstract
The utilization of glucose by the chemolithotroph Thiobacillus ferrooxidans results in a repression of the ability to oxidize iron, the substrate for autotrophic growth. An assay with resting cells was used to measure iron oxidation rates. Concomitant with the decreased iron oxidation rates, the enzyme responsible for carbon dioxide fixation, ribulose diphosphate (RuDP) carboxylase, was also repressed. Maximum iron oxidation rates precede peak RuDP carboxylase levels, consistent with the role of these processes in autotrophic metabolism in nonrepressed cells. The degree of iron oxidation repression depends on the organic substrate supplied, as does the level of RuDP carboxylase. The uptake of glucose parallels an increase in synthesis of glucose-6-phosphate dehydrogenase and the accumulation in cells of poly-β-hydroxybutyrate. The organism is also capable of growing on glucose and other organic supplements in the absence of its inorganic energy source; growth rates depend on the organic substrate supplied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleem M. I., Huang E. Carbon dioxide fixation and carboxydismutase in Thiobacillus novellus. Biochem Biophys Res Commun. 1965 Aug 16;20(4):515–520. doi: 10.1016/0006-291x(65)90610-8. [DOI] [PubMed] [Google Scholar]
- BRALEY S. A., Sr, KINSEL N. A., LEATHEN W. W. Ferrobacillus ferrooxidans: a chemosynthetic autotrophic Bacterium. J Bacteriol. 1956 Nov;72(5):700–704. doi: 10.1128/jb.72.5.700-704.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale N. L., Beck J. V. Evidence for the Calvin cycle and hexose monophosphate pathway in Thiobacillus ferrooxidans. J Bacteriol. 1967 Oct;94(4):1052–1059. doi: 10.1128/jb.94.4.1052-1059.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessie T., Neidhardt F. C. Adenosine triphosphate-linked control of Pseudomonas aeruginosa glucose-6-phosphate dehydrogenase. J Bacteriol. 1967 Apr;93(4):1337–1345. doi: 10.1128/jb.93.4.1337-1345.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London J., Rittenberg S. C. Effects of organic matter on the growth of Thiobacillus intermedius. J Bacteriol. 1966 Mar;91(3):1062–1069. doi: 10.1128/jb.91.3.1062-1069.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden B. A., Tu C. C. Regulation of autotrophic and heterotrophic carbon dioxide fixation in Hydrogenomonas facilis. J Bacteriol. 1967 Mar;93(3):886–893. doi: 10.1128/jb.93.3.886-893.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN M. P., LUNDGREN D. G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J Bacteriol. 1959 May;77(5):642–647. doi: 10.1128/jb.77.5.642-647.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., Korczynski M. S., Lundgren D. G. Kinetic studies of iron oxidation by whole cells of Ferrobacillus ferrooxidans. J Bacteriol. 1969 Aug;99(2):552–557. doi: 10.1128/jb.99.2.552-557.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafia F., Wilkinson R. F., Jr Growth of Ferrobacillus ferrooxidans on organic matter. J Bacteriol. 1969 Jan;97(1):256–260. doi: 10.1128/jb.97.1.256-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M., Margalith P., Lundgren D. G. Effect of glucose on carbon dioxide assimilation and substrate oxidation by Ferrobacillus ferrooxidans. J Bacteriol. 1967 Jun;93(6):1765–1769. doi: 10.1128/jb.93.6.1765-1769.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. Oxidation of elemental sulfur and sulfur compounds and CO2 fixation by Ferrobacillus ferrooxidans (Thiobacillus ferrooxidans). Can J Microbiol. 1970 Sep;16(9):845–849. doi: 10.1139/m70-142. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stukus P. E., DeCicco B. T. Autotrophic and heterotrophic metabolism of hydrogenomonas: regulation of autotrophic growth by organic substrates. J Bacteriol. 1970 Feb;101(2):339–345. doi: 10.1128/jb.101.2.339-345.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Silver M. The initial product and properties of the sulfur-oxidizing enzyme of thiobacilli. Biochim Biophys Acta. 1966 Jul 6;122(1):22–33. doi: 10.1016/0926-6593(66)90088-9. [DOI] [PubMed] [Google Scholar]
- Tabita R., Lundgren D. G. Heterotrophic metabolism of the chemolithotroph Thiobacillus ferrooxidans. J Bacteriol. 1971 Oct;108(1):334–342. doi: 10.1128/jb.108.1.334-342.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. S., Lundgren D. G. Poly-beta-hydroxybutyrate in the chemolithotrophic bacterium Ferrobacillus ferrooxidans. J Bacteriol. 1969 Feb;97(2):947–950. doi: 10.1128/jb.97.2.947-950.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


