Abstract
A cyclodextrin dimer has been synthesized with two β-cyclodextrins linked by a flexible chain containing a carbon–carbon double bond. This dimer binds and solubilizes a phthalocyanine-based photosensitizer that generates singlet oxygen on irradiation. When the complex is irradiated, the singlet oxygen cleaves the carbon–carbon link, and the cyclodextrins are released, liberating the photosensitizer into the light path. Ideas about how this phenomenon could be used to make photodynamic tumor therapy into a more selective process are described.
In photodynamic therapy, a dye such as a porphyrin or a phthalocyanine is used in conjunction with irradiation, e.g., by visible light. The excited-state dye converts triplet oxygen to the singlet, which is lethal to cells. Light directed into the area of a tumor can lead to the destruction of cancer cells (1, 2). One problem in this field is of course the accessibility of tumors to irradiation. Another problem has to do with the desirability of localizing the photosensitizer at the tumor site to prevent unwanted side reactions elsewhere. An approach to the latter problem has been to target the photosensitizer by using cancer-specific antibodies (3), but this use of a foreign protein may not be ideal.
A different approach has been proposed by Moser et al. (4). If a quite hydrophobic photosensitizer is bound to a cyclodextrin (CD) dimer, the complex will become much more hydrophilic, and the strongly bound sensitizer may not be easily taken up by tissues, including those that are not the target tumor cells. However, if the linker in the CD dimer can be cleaved by singlet oxygen, the photosensitizer should be released from the dimer and then enter nearby cells.
If this cleavage occurs in the tumor region that is being irradiated, there should be two results. First of all, cleavage of the CD dimer will deliver the photosensitizer to the affected cells, as proposed by Moser et al. (4). Perhaps more interestingly, the destruction of the dimer–sensitizer complex by light will cause more of it to diffuse into the irradiated region, concentrating the sensitizer where it is needed. In other words, the sensitizer will be concentrated into the directed light beam.
We have tested this concept by using an electron-rich carbon–carbon double bond as the group that is cleavable by singlet oxygen. The oxygen adds to the double bond to form a dioxetane, which fragments to form two carbonyl groups (5). Sulfur substituents at each end of the double bond increase its reactivity.
Materials and Methods
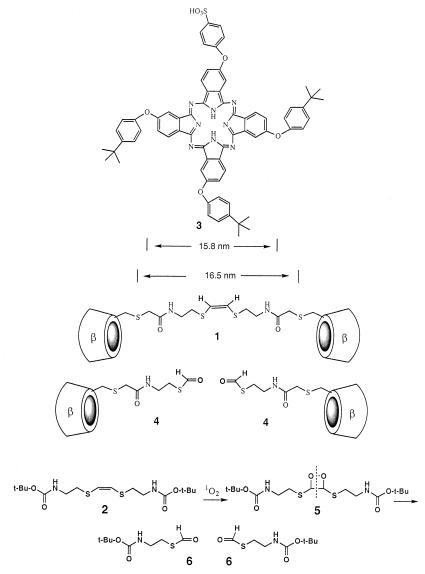
CD dimer 1 was synthesized by conversion of cystamine to its N-t-butoxycarbonyl derivative, then reduction of the disulfide link with sodium in ammonia and reaction of the thiolate with cis-1,2-dichloroethylene to form 2 (Scheme S1). All compounds were characterized by NMR and MS, and this olefin 2 had the characteristic infrared signal at 627 cm−1 for a cis double bond. Acid deprotection of 2 and then acylation with iodoacetic acid anhydride afforded a bis-iodide that was converted to a bis-thioacetate. Hydrolysis and then reaction of the bis-thiolate with 6-iodo-6-deoxy-β-CD and purification by reverse-phase chromatography afforded 1 as a white solid. It had 1H-NMR (300 MHz, DMSO-d-6): δ 8.09 (t, 2H), 6.22 (s, 2H), 5.6–5.9 (m, 28H), 4.75–4.95 (m, 14H), 4.40–4.55 (m, 12H), 3.45–3.90 (m, 56H), 2.77 (t, 4H). Fast atom bombardment MS: 2560 (M + 2).
Scheme 1.
Structures and the cleavage reaction.
Photosensitizer 3 (6) was received as a gift from D. Wöhrle (University of Bremen, Bremen, Germany), and additional samples were prepared according to his published procedure (6) by condensation of a mixture of 4-(4′-t-butylphenoxy)phthalonitrile and 4-phthalonitrile-4′-sulfonic acid in 3/1 proportions, followed by chromatographic purification.
Results and Discussion
The length of 1 in its extended conformation was calculated by using the program macromodel to be 16.5 nm between the two most distant sulfur atoms carrying the CD units; hence, we examined its complex with the phthalocyanine 3, carrying three tert-butylphenyl groups and one phenylsulfonate (6). Photosensitizer 3 has been reported to have λmax of 678 nm and a good quantum yield for singlet oxygen generation. Two of the tert-butylphenyl groups in 3 are attached to trans oxygen atoms that are calculated to be 15.8 nm apart, such that the tert-butylphenyl groups on opposite sides of 3 should fit nicely into the CDs of 1.
Compound 3 is so hydrophobic that it is quite insoluble in water, but it forms a complex with 1 that is water soluble. In our previous studies (7, 8) of the binding of dimers of β-CD to substrates with two tert-butylphenyl groups, we saw dissociation constants of the order of 10−8 M, whereas a tert-butylphenyl group binds into a simple β-CD with a dissociation constant of only 10−4 M. Thus, cleavage of the linker in the 1/3 complex should lead to dissociation of the CDs and release of insoluble 3 into the water medium. Although this result is indeed what we observe, the binding situation in the 1/3 complex is more complicated than our simple models predict.
The 1H-NMR of 1 shows a sharp singlet for the alkene protons at 6.2 ppm in DMSO solution, as expected for such a symmetric compound. However, in 2H2O solvent, the protons appear as an AB quartet at 6.0 and 6.2 ppm, but addition of hyodeoxycholic acid (which binds strongly into β-CD; ref. 9) to 1 causes collapse of the alkene quartet to a singlet at 6.2 ppm. The only sensible explanation of these findings is that the linking chain in 1 is partly tucked into one of the CDs in water solution (10, 11). Its binding is sufficiently strong that there is no equilibration of the buried end of the alkene with the other end, but it can be displaced by DMSO or hyodeoxycholic acid. That is, the hydrophobically bound chain equilibrates slowly on the NMR time scale in binding into the cavities of the two different CDs in water. Such competitive binding by the linker into one of the cavities will of course make the binding of 3 into 1 weaker. Also, if the dissociation of the 3/1 complex leads to precipitation of 3, this binding will also make the apparent binding weaker.
We have carried out a determination of the binding constant for the 3/1 complex by competitive binding of the fluorescent dye N-(p-tert-butylphenyl)-2-aminonaphthalene-6-sulfonate (BNS) into 1. BNS is fluorescent when bound into a hydrophobic cavity but only weakly fluorescent in water solution; we have used competitive binding between BNS and a nonfluorescent substrate previously to determine strong binding constants (7, 8). From the slope of fluorescence intensity vs. BNS concentration when it was titrated into a solution of 1 (3.3 × 10−7 M), we could determine the binding constant of BNS to 1 as 1.9 × 105 M−1 and compare it with the result when BNS was titrated into the 3/1 complex at the same 3.3 × 10−7 M concentration. From this comparison, the binding constant for 3 to 1 was found to be 2 × 106 M−1, 50 times less than the 108 M−1 estimate based on our previous studies (7, 8).
Of course this decrease might reflect the insolubility of 3, but we see no precipitate during the titration at this concentration. The NMR evidence that some of the flexible linker chain is bound into one of the CDs is a more likely explanation, because this arrangement should lower the binding of 3 into 1 (10). When the chain is cleaved, the cavities of both CDs in 4 should have affinities for 3 lower than 104 M−1. Each has a hydrophobic chain linked to it that should bind back into the CD cavity and contribute to the dissociation of 3 after chain cleavage. Confirming this hypothesis, a compound analogous to 4 with a methyl group replacing the labile formyl group has an affinity for p-tert-butylbenzoic acid of only 1.7 × 103 M−1 in water, an order of magnitude lower than the affinity of simple β-CD. Thus, the effect of chain binding into a CD cavity is to reinforce the expectation that singlet oxygen will cause precipitation of 3.
Singlet oxygen was generated by bubbling oxygen through a solution of olefin 2 (7 mM) with methylene blue (7 μM) in acetonitrile while irradiating it with a halogen lamp with a 540-nm cutoff filter. Compound 2 was cleaved to the thioformate 6 (1H-NMR signal at 8.3 ppm, correct mass spectrum) via the dioxetane 5. When a solution of the 1/3 complex in water was irradiated with strong white light, the insoluble 3 precipitated, while the 1H-NMR signal for the olefinic protons in 1 was replaced by a signal at 8.3 ppm for the thioformate. When methylene blue was used as the photosensitizer in solution with dimer 1, there was also cleavage of 1 to the thioformate, but additional 1H-NMR peaks were seen—apparently from singlet oxygen reaction in other areas of 1, including a signal at 10.2 ppm that we ascribe to oxidation of a CD hydroxymethyl group to an aldehyde—that had not appeared in the photoreaction of complex 1/3. Thus, the singlet oxygen generated by phthalocyanine 3 in its complex with 1 is apparently being directed to the double bond of 1 because of the geometry of the 1/3 complex (12, 13).
In an additional experiment, a solution of the 1/3 complex in water was irradiated through a hole in a shield surrounding a small tube. The precipitate of 3 was seen only opposite the hole, with the rest of the tube remaining clear. Thus, the liberation of 3 is indeed restricted to the area exposed to light.
Conclusions
It remains to be seen whether this approach to photodynamic tumor therapy has clinical advantages. However, the original proposal (4) that a CD dimer with a linker cleavable by singlet oxygen would be able to release a bound hydrophobic photosensitizer in the region in which light is focused has been validated by these experiments.
Acknowledgments
We thank Prof. D. Wöhrle for gifts of phthalocyanine 3. This work was supported by the National Institutes of Health. A.R. thanks the Heinrich-Hertz Foundation of North Rhine-Westphalia for a postdoctoral fellowship.
Abbreviations
- CD
cyclodextrin
- BNS
N-(p-tert-butylphenyl)-2-aminonaphthalene-6-sulfonate
References
- 1.Dougherty T J, Gomer C J, Henderson B W, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wöhrle D, Hirth A, Bogdahn-Rai T, Schnurpfeil G, Shopova M. Russ Chem Bull. 1998;47:807–816. [Google Scholar]
- 3.Ruebner A, Kirsch D, Andress S, Decker W, Roeder B, Spengler B, Kaufmann R, Moser J G. J Inclusion Phenom. 1997;27:69–84. [Google Scholar]
- 4.Moser J G, Ruebner A, Vervoorts A, Wagner B. In: Proceedings of the Eighth International Symposium on Cyclodextrins. Szetli J, editor. Boston: Kluwer; 1996. pp. 71–76. [Google Scholar]
- 5.Sauter M, Adam W. Acc Chem Res. 1995;28:289–298. [Google Scholar]
- 6.Kliesch H, Weitemeyer A, Müller S, Wöhrle D. Liebigs Ann. Chem. 1995. 1269–1273. [Google Scholar]
- 7.Breslow R, Greenspoon N, Guo T, Zarzycki R. J Am Chem Soc. 1989;111:8296–8297. [Google Scholar]
- 8.Breslow R, Halfon S, Zhang B. Tetrahedron. 1995;51:377–388. [Google Scholar]
- 9.Yang Z, Breslow R. Tetrahedron Lett. 1997. 6171–6172. [Google Scholar]
- 10.Venema F, Baselier C M, Feiters M C, Nolte R J M. Tetrahedron Lett. 1994. 8661–8664. [Google Scholar]
- 11.Birlirakis N, Benoit H, Berthault P. Tetrahedron. 1998;54:3513–3522. [Google Scholar]
- 12.Breslow E, Koehler R, Girotti A W. J Biol Chem. 1967;242:4149–4156. [PubMed] [Google Scholar]
- 13.Neckers D C, Paczkowski J. J Am Chem Soc. 1986;108:291–292. [Google Scholar]