Abstract
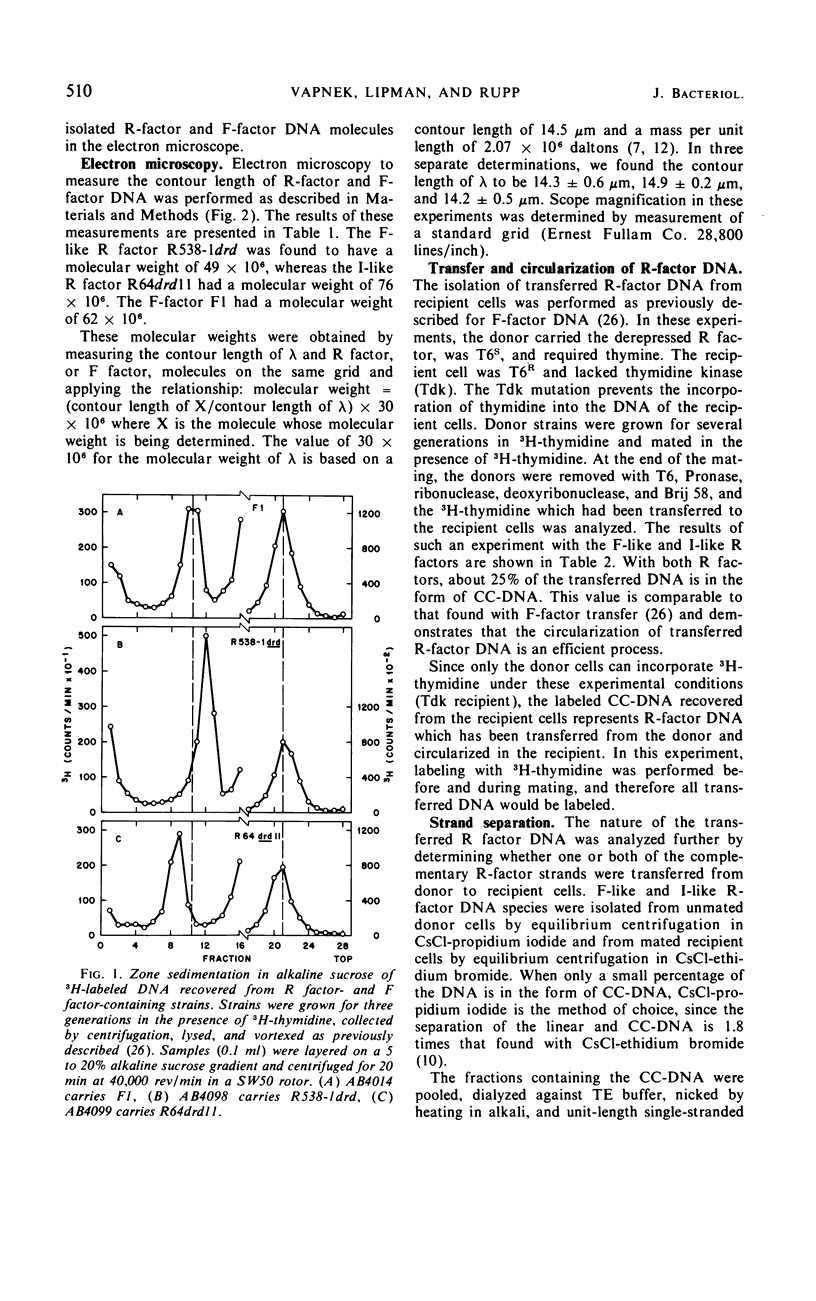
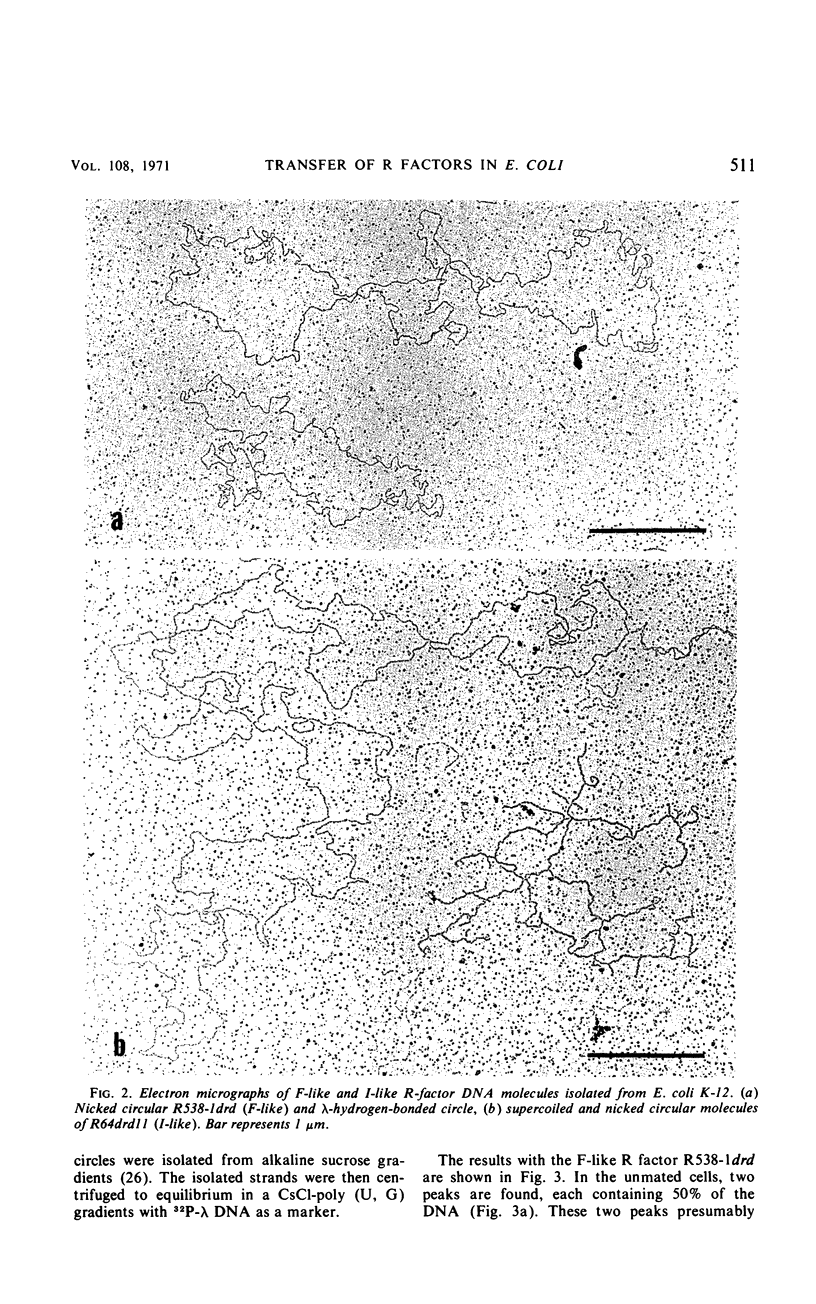
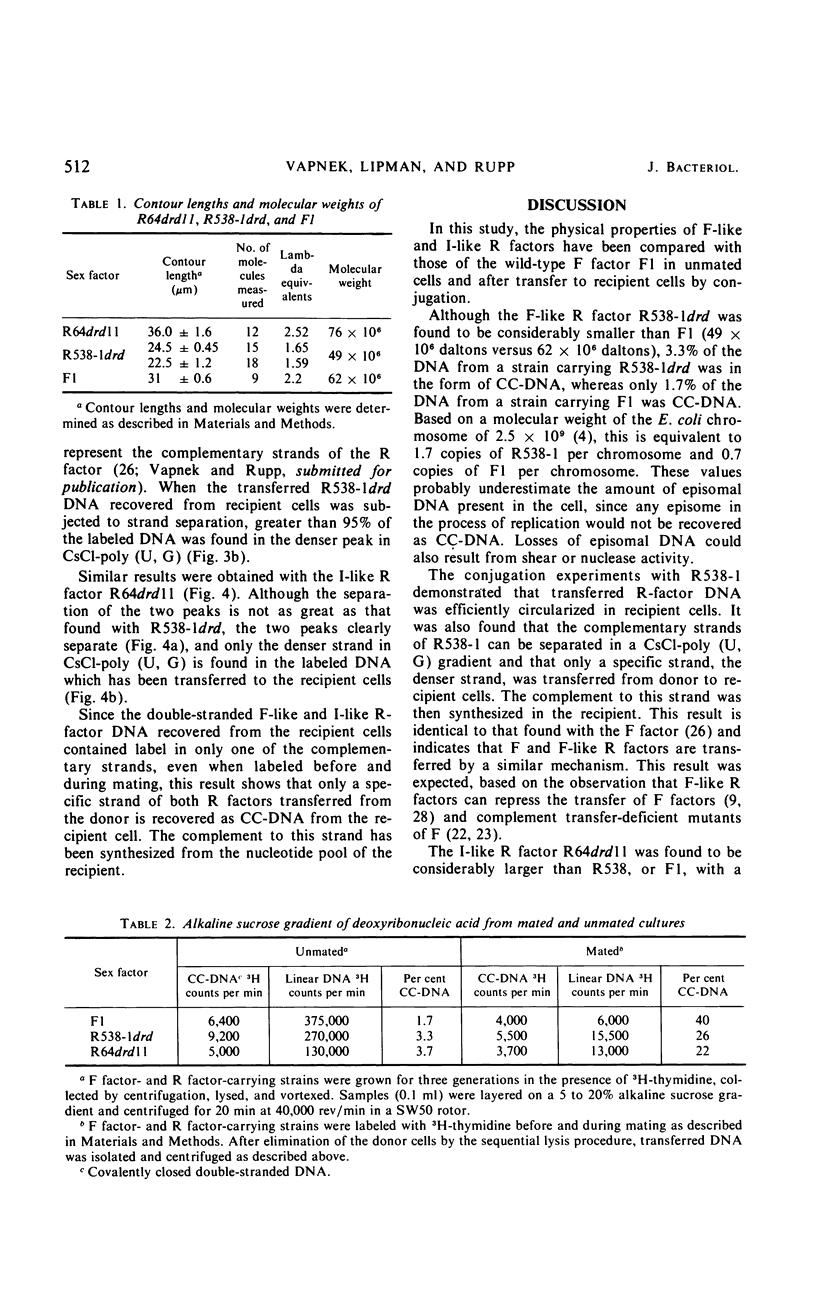
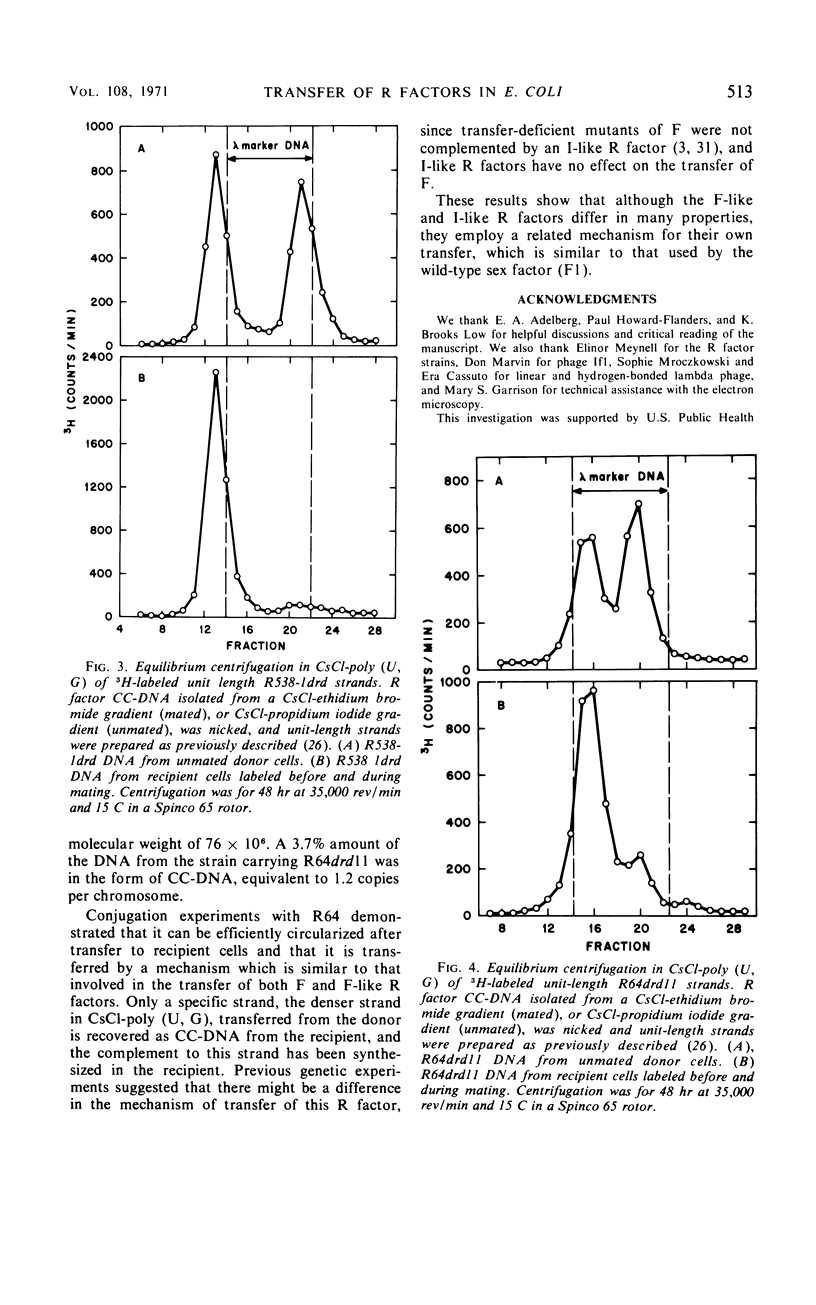
The physical properties of F-like and I-like R factors have been compared with those of the wild-type F factor in Escherichia coli K-12 unmated cells and after transfer to recipient cells by conjugation. The F-like R factor R538-1drd was found to have a molecular weight of 49 × 106, whereas the molecular weight of the I-like R factor R64drd11 was 76 × 106. The wild-type F factor, F1, had a molecular weight of 62 × 106. When conjugation experiments are performed by using donor strains carrying these derepressed F-like or I-like R factors, the transferred deoxyribonucleic acid can be isolated as a covalently closed circle from the recipient cells. This circular deoxyribonucleic acid was characterized by making use of the observation that the complementary strands of these R factors can be separated in a CsCl-poly (U, G) equilibrium gradient. The results of the strand-separation experiments show that only one of the complementary strands of the R factor is transferred from the donor to the recipient. With both the F-like and I-like R factors, this strand is the heavier strand in CsCl-poly (U, G). These results indicate that even though F-like and I-like R factors differ greatly in many properties (phage specificity, size, compatability, etc.), they are transferred by a similar mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cooke M., Meynell E., Lawn A. M. Mutant Hfr strains defective in transfer: restoration by F-like and I-like de-repressed R factors. Genet Res. 1970 Aug;16(1):101–112. doi: 10.1017/s0016672300002317. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Datta N., Lawn A. M., Meynell E. The relationship of F type piliation and F phage sensitivity to drug resistance transfer in R+F- Escherichia coli K 12. J Gen Microbiol. 1966 Nov;45(2):365–376. doi: 10.1099/00221287-45-2-365. [DOI] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. I. Specific labeling of F'Lac DNA. J Mol Biol. 1968 Feb 28;32(1):15–23. doi: 10.1016/0022-2836(68)90141-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Molecular weights of coliphages and coliphage DNA. IV. Molecular weights of DNA from bacteriophages T4, T5 and T7 and the general problem of determination of M. J Mol Biol. 1970 Dec 28;54(3):567–577. doi: 10.1016/0022-2836(70)90127-0. [DOI] [PubMed] [Google Scholar]
- HIROTA Y., NISHIMURA Y., ORSKOV F., ORSKOV I. EFFECT OF DRUG-RESISTANCE FACTOR R ON THE F PROPERTIES OF ESCHERICHIA COLI. J Bacteriol. 1964 Feb;87:341–351. doi: 10.1128/jb.87.2.341-351.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontomichalou P., Mitani M., Clowes R. C. Circular R-factor molecules controlling penicillinase synthesis, replicating in Escherichia coli under either relaxed or stringent control. J Bacteriol. 1970 Oct;104(1):34–44. doi: 10.1128/jb.104.1.34-44.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell E. Serotypes of sex pili. J Hyg (Lond) 1970 Dec;68(4):683–694. doi: 10.1017/s0022172400042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Meynell G. G., Meynell E., Datta N. Sex pili and the classification of sex factors in the enterobacteriaceae. Nature. 1967 Oct 28;216(5113):343–346. doi: 10.1038/216343a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. The relation of resistance transfer factors to the F-factor (sex-factor) of Escherichia coli K12. Genet Res. 1966 Feb;7(1):134–140. doi: 10.1017/s0016672300009538. [DOI] [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Filamentous phages specific for the I sex factor. Nature. 1968 Mar 23;217(5134):1184–1186. doi: 10.1038/2171184a0. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Sex pili and common pili in the conjugational transfer of colicin factor Ib by Salmonella typhimurium. Genet Res. 1967 Jun;9(3):359–367. doi: 10.1017/s0016672300010636. [DOI] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. C. Molecular recombination between R-factor deoxyribonucleic acid molecules in Escherichia coli host cells. J Bacteriol. 1970 Jul;103(1):166–177. doi: 10.1128/jb.103.1.166-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E., Nishimura Y., Hirota Y. Transfer-defective mutants of sex factors in Escherichia coli. I. Defective mutants and complementation analysis. Genetics. 1970 Feb;64(2):173–188. doi: 10.1093/genetics/64.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E. Transfer-defective mutants of sex factors in Escherichia coli. II. Deletion mutants of an F-prime and deletion mapping of cistrons involved in genetic transfer. Genetics. 1970 Feb;64(2):189–197. doi: 10.1093/genetics/64.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGINO Y., HIROTA Y. Conjugal fertility associated with resistance factor R in Escherichia coli. J Bacteriol. 1962 Nov;84:902–910. doi: 10.1128/jb.84.5.902-910.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Asymmetric segregation of the complementary sex-factor DNA strands during conjugation in Escherichia coli. J Mol Biol. 1970 Nov 14;53(3):287–303. doi: 10.1016/0022-2836(70)90066-5. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae IV. Interactions between resistance transfer factor and F-factor in Escherichia coli K-12. J Bacteriol. 1962 Apr;83:727–735. doi: 10.1128/jb.83.4.727-735.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Takano T., Arai T., Nishida H., Sato S. Episome-mediated Transfer of Drug Resistance in Enterobacteriaceae X. Restriction and Modification of Phages by fi R Factors. J Bacteriol. 1966 Aug;92(2):477–486. doi: 10.1128/jb.92.2.477-486.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. The interaction of an I-like R factor and transfer-deficient mutants of Flac in E. coli K 12. Mol Gen Genet. 1970;108(4):365–373. doi: 10.1007/BF00267774. [DOI] [PubMed] [Google Scholar]



