Abstract
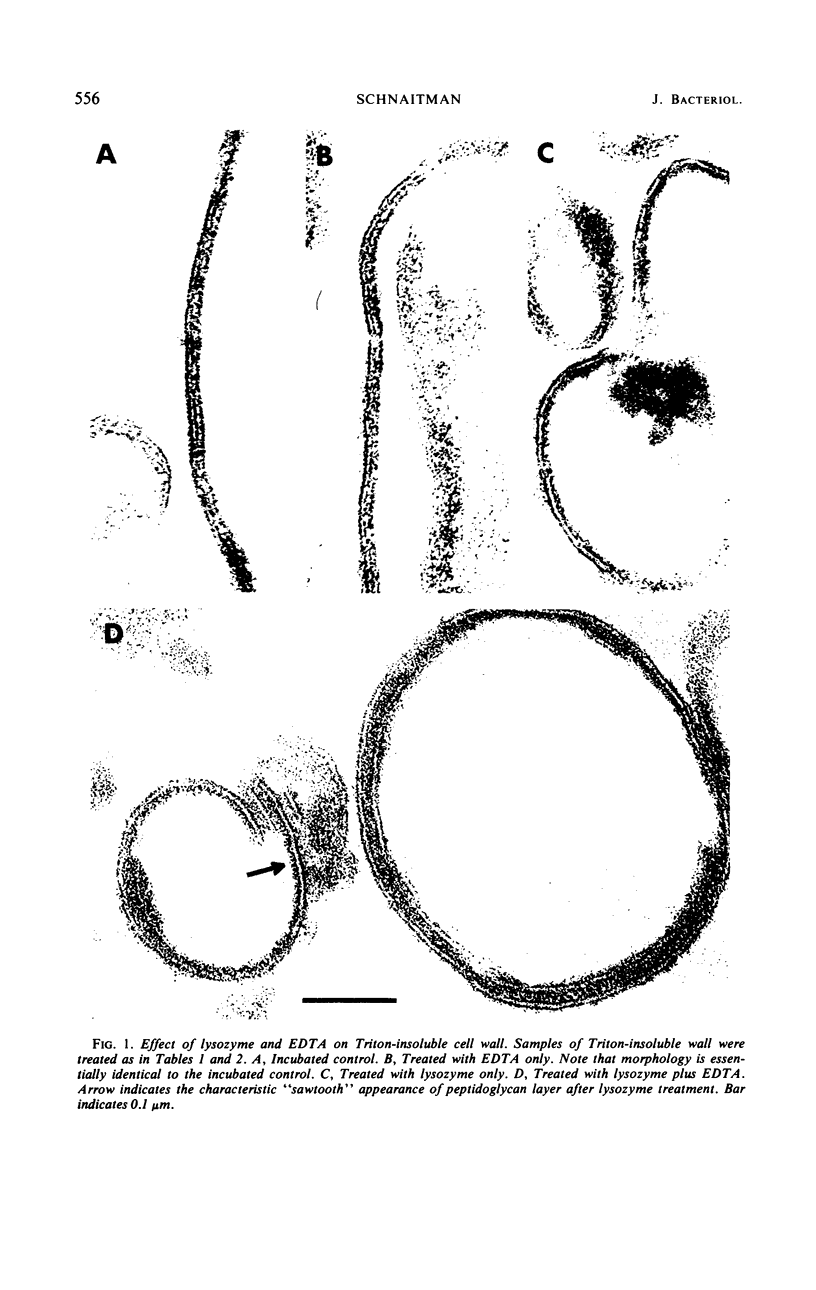
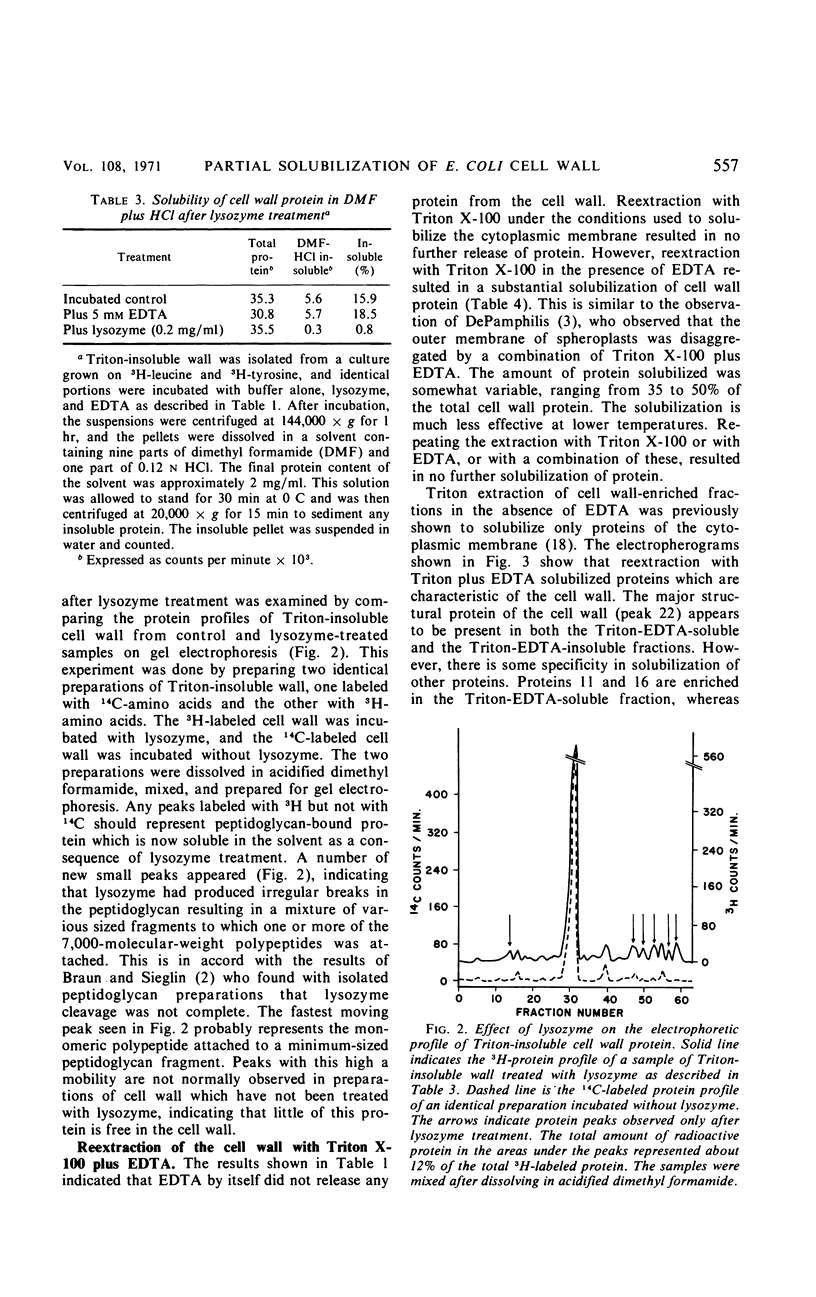
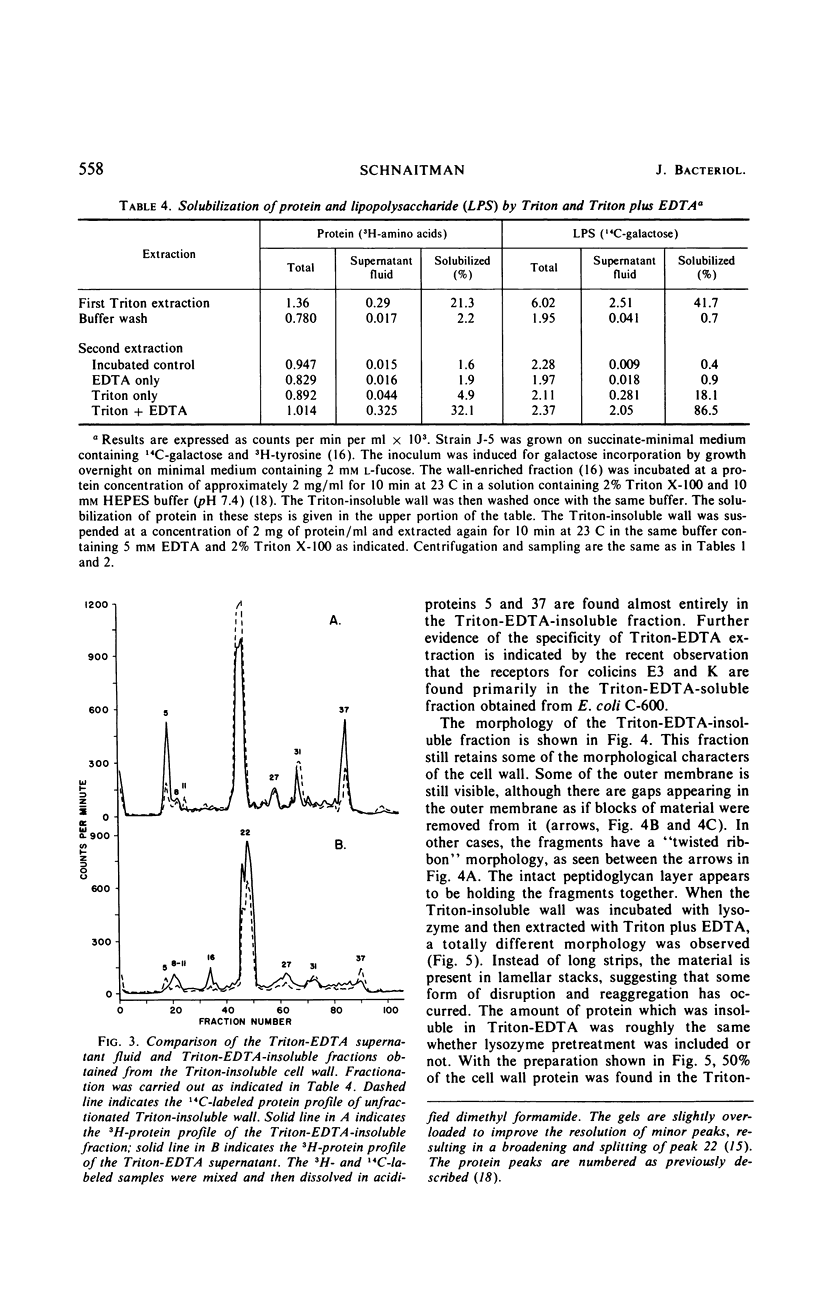
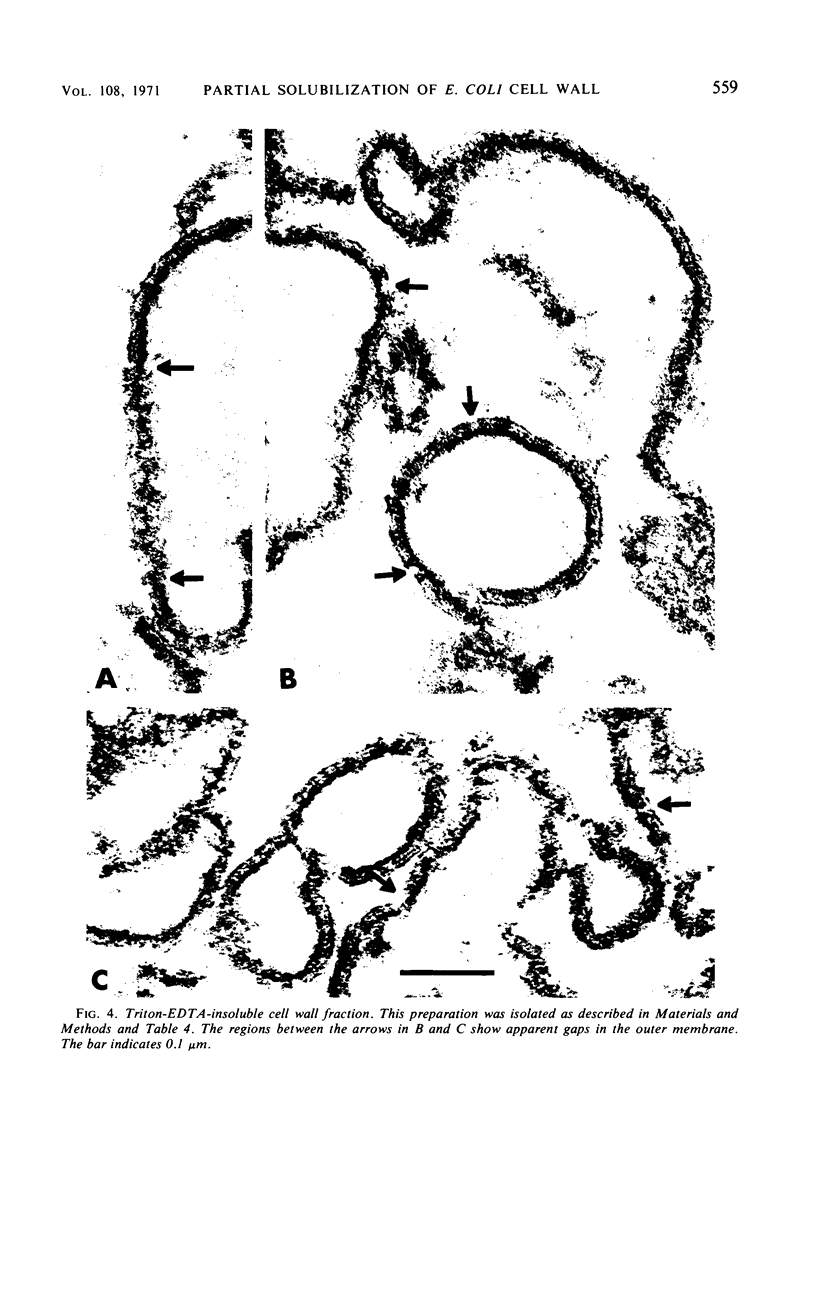
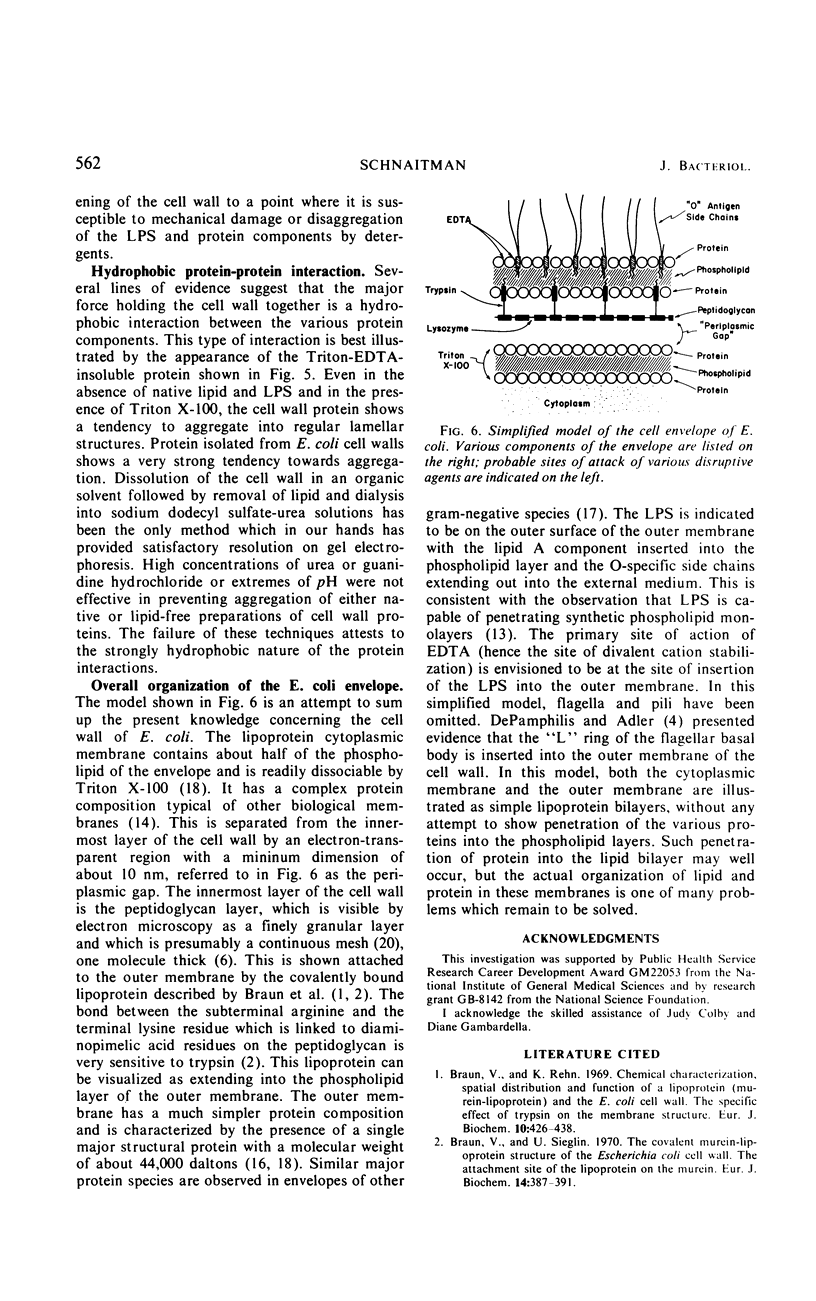
Extraction of a partially purified preparation of cell walls from Escherichia coli with the nonionic detergent Triton X-100 removed all cytoplasmic membrane contamination but did not affect the normal morphology of the cell wall. This Triton-treated preparation, termed the “Triton-insoluble cell wall,” contained all of the protein of the cell wall but only about half of the lipopolysaccharide and one-third of the phospholipid of the cell wall. This Triton-insoluble cell wall preparation was used as a starting material in an investigation of several further treatments. Reextraction of the Triton-insoluble cell wall with either Triton X-100 or ethylenediaminetetraacetic acid (EDTA) caused no further solubilization of protein. However, when the Triton-insoluble cell wall was extracted with a combination of Triton X-100 and EDTA, about half of the protein and all of the remaining lipopolysaccharide and phospholipid were solubilized. The material which remained insoluble after this combined Triton and EDTA extraction still retained some of the morphological features of the intact cell wall. Treatment of the Triton-insoluble cell wall with lysozyme resulted in a destruction of the peptidoglycan layer as seen in the electron microscope and in a release of diaminopimelic acid from the cell wall but did not solubilize any cell wall protein. Extraction of this lysozyme-treated preparation with a combination of Triton X-100 and EDTA again solubilized about half of the cell wall protein but resulted in a drastic change in the morphology of the Triton-EDTA-insoluble material. After this treatment, the insoluble material formed lamellar structures. These results are interpreted in terms of the types of noncovalent bonds involved in maintaining the organized structure of the cell wall and suggest that the main forces involved are hydrophobic protein-protein interactions between the cell wall proteins and to a lesser degree a stabilization of protein-protein and protein-lipopolysaccharide interactions by divalent cations. A model for the structure of the E. coli cell wall is presented.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Wolff H. The murein-lipoprotein linkage in the cell wall of Escherichia coli. Eur J Biochem. 1970 Jun;14(2):387–391. doi: 10.1111/j.1432-1033.1970.tb00301.x. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L. Dissociation and reassembly of Escherichia coli outer membrane and of lipopolysaccharide, and their reassembly onto flagellar basal bodies. J Bacteriol. 1971 Mar;105(3):1184–1199. doi: 10.1128/jb.105.3.1184-1199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer S., Fleischer B., Stoeckenius W. Fine structure of lipid-depleted mitochondria. J Cell Biol. 1967 Jan;32(1):193–208. doi: 10.1083/jcb.32.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy S. B., Leive L. An equilibrium between two fractions of lipopolysaccharide in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1435–1439. doi: 10.1073/pnas.61.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. E., Bruns R. R. Structural modulations of plasmalemmal vesicles. J Cell Biol. 1968 Jun;37(3):633–649. doi: 10.1083/jcb.37.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of rat liver mitochondrial and microsomal membrane proteins. Proc Natl Acad Sci U S A. 1969 Jun;63(2):412–419. doi: 10.1073/pnas.63.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Comparison of the envelope protein compositions of several gram-negative bacteria. J Bacteriol. 1970 Dec;104(3):1404–1405. doi: 10.1128/jb.104.3.1404-1405.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shands J. W. Localization of Somatic Antigen on Gram-Negative Bacteria by Electron Microscopy. J Bacteriol. 1965 Jul;90(1):266–270. doi: 10.1128/jb.90.1.266-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]