Abstract
With the postgenome era rapidly approaching, new strategies for the functional analysis of proteins are needed. To date, proteomics efforts have primarily been confined to recording variations in protein level rather than activity. The ability to profile classes of proteins on the basis of changes in their activity would greatly accelerate both the assignment of protein function and the identification of potential pharmaceutical targets. Here, we describe the chemical synthesis and utility of an active-site directed probe for visualizing dynamics in the expression and function of an entire enzyme family, the serine hydrolases. By reacting this probe, a biotinylated fluorophosphonate referred to as FP-biotin, with crude tissue extracts, we quickly and with high sensitivity detect numerous serine hydrolases, many of which display tissue-restricted patterns of expression. Additionally, we show that FP-biotin labels these proteins in an activity-dependent manner that can be followed kinetically, offering a powerful means to monitor dynamics simultaneously in both protein function and expression.
Serine hydrolases play important roles in numerous developmental and tissue-specific events in vivo, including blood coagulation (1), inflammation (2), angiogenesis (3), neural plasticity (4), peptide hormone processing (5), and T-lymphocyte-mediated cytotoxicity (6). Additionally, several human diseases are associated with dysfunctions in serine proteases and/or their endogenous inhibitory proteins, including hemorrhagic disorders (7), emphysema (7), and cancer (8). The large number of mammalian serine hydrolases identified to date is both impressive and perplexing, with the endogenous functions of many members of this enzyme family remaining unknown. As ORFs encoding putative serine hydrolases continue to accumulate in public databases (9–11), the need for alternative experimental methods to study these enzymes is evident. One attractive approach for the analysis of serine hydrolase function would be to characterize these enzymes collectively, rather than individually. In particular, the majority of serine hydrolases are potently and irreversibly inhibited by fluorophosphonate/fluorophosphate (FP) derivatives like diisopropyl fluorophosphate (12, 13), whereas cysteine, aspartyl, and metallohydrolases are for the most part inert to such agents. Moreover, the reactivity of FPs with serine hydrolases requires that the enzymes be in a catalytically active state (12–14). Accordingly, we hypothesized that an FP linked to a small molecule reporter group might serve as a potent and selective probe for monitoring simultaneously the activities of multiple serine hydrolases. In this manner, serine hydrolases could be visualized on a systems level of analysis, greatly accelerating the assignment of potential functions and malfunctions to members of this enzyme family. Here, we report the synthesis and characterization of a biotinylated long-chain fluorophosphonate, referred to as FP-biotin and highlight its utility as an agent for profiling dynamics in serine hydrolase expression and function.
Experimental Procedures
Chemical Synthesis of FP-Biotin.
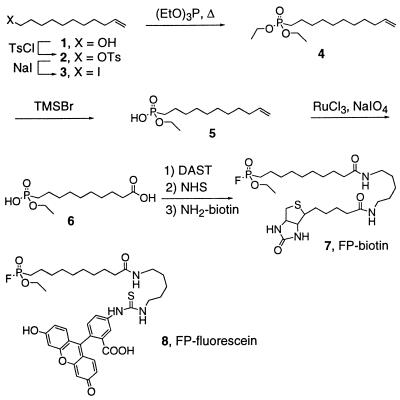
Compound numbers in bold refer to structures shown in Scheme S1.
Scheme 1.
Route for the synthesis of FP-biotin.
1-[(p-Toluenesulfonyl)oxy]-10-undecene (2).
A solution of 1 [2.0 g, 11.8 mmol, 1.0 equivalent (equiv)] in pyridine (14.0 ml, 177 mmol, 15 equiv) was cooled to 0°C and treated with p-toluenesulfonyl chloride (pTsCl) (4.5 g, 23.6 mmol, 2.0 equiv). The reaction mixture was kept at 0°C for 10 h and then partitioned between ethyl acetate (200 ml) and water (200 ml). The organic layer was washed with 10% aqueous HCl (2 × 200 ml) and saturated aqueous NaCl (200 ml), dried (Na2SO4), and concentrated under reduced pressure. Chromatography (SiO2, 5 × 15 cm, 2% ethyl acetate-hexanes) afforded 2 (3.6 g, 3.8 g theoretical, 94%) as a colorless oil: 1H NMR (CDCl3, 250 MHz) δ 7.76 (d, J = 6.5 Hz, 2H, ArH), 7.32 (d, J = 7.3 Hz, 2H, ArH), 5.95–5.75 (m, 1H, RCH ⩵ CH2), 5.03–4.90 (m, 2H, RCH ⩵ CH2), 3.98 (t, J = 6.5 Hz, 2H, CH2OTs), 2.42 (s, 3H, ArCH3), 2.02 (m, 2H, CH2CH ⩵ CH2), 1.65 (p, J = 6.9 Hz, 2H, CH2CH2OTs), 1.50–1.20 (m, 12H); matrix-assisted laser desorption ionization Fourier transform MS (MALDI-FTMS) (2,5-dihydroxybenzoic acid, DHB) m/z 347.1657 (C18H28O3S + Na+ requires 347.1658).
1-Iodo-10-undecene (3).
A solution of 2 (3.4 g, 10.5 mmol, 1.0 equiv) in acetone (21 ml, 0.5 M) was treated with NaI (3.2 g, 21 mmol, 2.0 equiv), and the reaction mixture was stirred at reflux for 2 h, producing a yellow-orange solution. The reaction mixture was then partitioned between ethyl acetate (200 ml) and water (200 ml). The organic layer was washed sequentially with saturated aqueous Na2S2O3 (100 ml) and saturated aqueous NaCl (100 ml), dried (Na2SO4), and concentrated under reduced pressure. Chromatography (SiO2, 5 × 15 cm, 1–2% ethyl acetate-hexanes) afforded 3 (2.3 g, 2.9 g theoretical, 78%) as a colorless oil: 1H NMR (CDCl3, 250 MHz) δ 5.95–5.75 (m, 1H, RCH ⩵ CH2), 5.03–4.90 (m, 2H, RCH ⩵ CH2), 3.16 (t, J = 7.0 Hz, 2H, CH2I), 2.02 (m, 2H, CH2CH ⩵ CH2), 1.80 (p, J = 6.9 Hz, 2H, CH2CH2I), 1.50–1.20 (m, 12H).
1-[Bis(ethoxy)phosphinyl]-10-undecene (4).
Triethylphosphite (12.2 ml, 71 mmol, 10 equiv) was added to 3 (2.0 g, 7.1 mmol, 1.0 equiv), and the mixture was stirred at reflux for 15 h. The excess triethylphosphite was removed by distillation and the remaining residue submitted to flash chromatography (SiO2, 5 × 15 cm, 25–50% ethyl acetate-hexanes gradient elution) to afford 4 (1.30 g, 2.1 g theoretical, 62%) as a colorless oil: 1H NMR (CDCl3, 250 MHz) δ 5.95–5.75 (m, 1H, RCH ⩵ CH2), 5.03–4.90 (m, 2H, RCH ⩵ CH2), 4.05 (m, 4H, CH3CH2OP), 2.02 (m, 2H, CH2CH ⩵ CH2), 1.80–1.20 (m, 20H); MALDI-FTMS (DHB) m/z 291.2088 (C15H31O3P + H+ requires 291.2089).
1-(Ethoxyhydroxyphosphinyl)-10-undecene (5).
A solution of compound 4 (0.31 g, 1.07 mmol, 1.0 equiv) in CH2Cl2 (4.0 ml, 0.3 M) was treated dropwise with trimethylsilyl bromide (0.17 ml, 1.28 mmol, 1.2 equiv). The reaction was stirred at 25°C for 1 h, quenched with 5 ml of 5% [wt/vol] KHSO4, and stirred vigorously for 5 min. The reaction mixture was then partitioned between ethyl acetate (100 ml) and water (100 ml), and the organic layer was washed with saturated aqueous NaCl (200 ml), dried (Na2SO4), and concentrated under reduced pressure. Chromatography (SiO2, 2 × 8 cm, 12–20% CH3OH-CHCl3 with 1% aqueous NH4OH) afforded 5 (0.10 g, 0.28 g theoretical, 36.2.%; most of the remaining mass was recovered as starting material) as a clear oil: 1H NMR (CDCl3, 250 MHz) δ 5.95–5.75 (m, 1H, RCH ⩵ CH2), 5.03–4.90 (m, 2H, RCH ⩵ CH2), 4.05 (m, 2H, CH3CH2OP), 2.02 (m, 2H, CH2CH ⩵ CH2), 1.80–1.20 (m, 20H); MALDI-FTMS (DHB) m/z 285.1589 (C13H27O3P + Na+ requires 285.1596).
10-(Ethoxyhydroxyphosphinyl)decanoic acid (6).
Compound 5 (0.10 g, 0.38 mmol, 1.0 equiv) in a biphasic solution composed of CCl4-CH3CN-H2O (1.0 ml-1.0 ml-1.5 ml; total volume of 3.5 ml, 0.11 M) was treated sequentially with sodium periodate (0.31 g, 1.56 mmol, 4.1 equiv) and ruthenium trichloride hydrate (0.002 g, 0.009 mmol, 0.022 equiv). The reaction mixture was stirred at 25°C for 2 h and then partitioned between CH2Cl2 (50 ml) and 1 N aqueous HCl (50 ml). The organic layer was washed with saturated aqueous NaCl (25 ml), dried (Na2SO4), and concentrated under reduced pressure. The resulting residue was resuspended in 40 ml of diethyl ether, filtered through a Celite pad, and concentrated under reduced pressure to afford 6 (0.09 g, 0.11 g theoretical, 83%) as a colorless semisolid: 1H NMR (CDCl3, 250 MHz) δ 4.05 (m, 2H, CH3CH2OP), 2.32 (t, J = 7.5 Hz, 2H, CH2COOH), 1.80–1.20 (m, 16H); fast-atom bombardment high-resolution MS (FABHRMS) (NBA-NaI) m/z 303.1340 (C12H25O5P + Na+ requires 303.1337).
FP-biotin, or 10-(fluoroethoxyphosphinyl)-N-(biotinamidopentyl)decanamide (7).
A solution of 6 (0.007 g, 0.025 mmol, 4.0 equiv) in CH2Cl2 (0.4 ml, 0.06 M) at −78°C was treated dropwise with (diethylamino)sulfur trifluoride (0.021 ml, 0.10 mmol, 4.0 equiv), brought to 25°C, and stirred for 5 min. The reaction mixture was then treated with one-half reaction volume of dimethyl formamide containing N-hydroxysuccinimide (0.05 g, 0.25 mmol, 10 equiv) and stirred for an additional 10 min at 25°C. The reaction mixture was partitioned between ethyl acetate (50 ml) and water (50 ml), and the organic layer was washed with saturated aqueous NaCl (200 ml), dried (Na2SO4), and concentrated under reduced pressure to afford 10-(fluoroethoxyphosphinyl)-N-(hydroxysuccinyl)decanamide (as judged by crude 1H NMR; data not shown). Without further purification, this compound was treated with 5-(biotinamido)-pentylamine (Pierce, 0.0021 g, 0.062 mmol, 1.0 equiv) in MeOH (0.02 ml) and stirred for 10 min. The solvent was evaporated under a stream of gaseous nitrogen, and the remaining residue was washed sequentially with diethyl ether and ethyl acetate, solubilized in a minimal volume of chloroform, transferred to a clean glass vial and the solvent evaporated. This process was repeated once more to rid the desired biotinylated product of excess reagents and byproducts, affording 7 as a white film (0.0011 g, 0.0038 g theoretical, 29%): 1H NMR (CDCl3, 400 MHz) δ 5.98 (b s, 1H, NH), 5.83 (b s, 1H, NH), 5.60 (b s, 1H, NH), 4.90 (b s, 1H, NH), 4.51 (m, 1H), 4.32 (m, 1H), 4.27 (m, 2H, CH3CH2OP), 3.22 (m, 4H, CH2NHCOR), 3.15 (m, 1H), 2.92 (dd, J = 4.9 and 12.9 Hz, 1H), 2.72 (d, J = 12.9 Hz, 1H), 2.20 (m, 4H, CH2CONHR), 1.85–1.24 (m, 31H); FABHRMS (NBA-NaI) m/z 593.3319 (C27FH50N4O5PS + H+ requires 593.3302).
Preparation of Tissue Samples for Reaction with FP-Biotin.
Rat tissues were Dounce-homogenized in Tris buffer (50 mM Tris⋅HCl buffer, pH 8.0/0.32 M sucrose). Tissue extracts were centrifuged sequentially at 1,100 × g (5 min), 22,000 × g (30 min), and 105,000 × g (60 min). The final supernatant (soluble fraction) was adjusted to 1 mg protein/ml and then incubated for 30 min at 4°C with one-tenth volume of avidin-agarose (Sigma) to deplete endogenous avidin-binding proteins. After a brief spin to pellet the avidin beads (2 min at 10,000 × g), the soluble fraction was removed and treated with FP-biotin, as described below.
Reaction of Protein Samples with FP-Biotin.
Unless otherwise indicated, reactions between protein samples and FP-biotin were conducted as follows: FP-biotin (0.4 nmol) in CHCl3 was added to a glass vial and the solvent evaporated under a stream of gaseous nitrogen. Ethanol (7.5 μL) was added to the vial, followed immediately by 192.5 μl of a 1 μg/μl protein stock in Tris buffer, and the reaction mixture was incubated at 25°C for 30 min (final concentration of FP-biotin was 2 μM). The reaction mixture was quenched by adding 1 vol equiv of standard 2× SDS/PAGE loading buffer (reducing) and heating the sample at 80°C for 5 min. Reactions conducted for longer times (1 hr) or with higher concentrations of FP-biotin (20 μM) did not produce significant increases in the labeling intensity of most proteins, indicating that the majority of proteins had reacted to completion under the reported conditions. However, reactions with higher concentrations of FP-biotin did begin to show significant levels of nonspecific labeling (defined as the appearance of new protein bands that reacted with FP-biotin in both preheated and unheated samples). More recent experiments have indicated that FP-biotin can be stored as a stock solution in DMSO at −20°C and then added directly to reactions with protein extracts to produce data equivalent to those described in this manuscript.
Detection of FP-Biotin-Reactive Proteins by SDS/PAGE–Western Blotting.
Quenched FP-biotin reactions were run on SDS/PAGE (10 μg protein/gel lane) and transferred by electroblotting onto nitrocellulose membranes, which were blocked in Tris-buffered saline (TBS) with 1% Tween (TBS-Tween) and 3% (wt/vol) nonfat dry milk for either 1 h at 25°C or overnight at 4°C. Blots were then treated with an avidin-horseradish peroxidase conjugate (Bio-Rad, 1:2,000 dilution) in TBS-Tween with 1% nonfat dry milk for 30 min at 25°C. The blot was washed with TBS-Tween three times (10 min/wash), treated with SuperSignal chemiluminescence reagents (Bio-Rad), and exposed to film for 0.1 to 8 min before development. For the comparison of soybean trypsin inhibitor (STI)-treated vs. untreated protein samples, the relative amounts of FP-biotin labeling were estimated by film densitometry by using an AlphaImager 2000 (Alpha Innotech, San Leandro, CA).
Molecular Characterization of FP-Biotin-Reactive Proteins.
Brain-soluble fractions were run over a Q Sepharose column by using an ÄKTA FPLC (Amersham Pharmacia Biotech) and eluted with a linear gradient of 0–500 mM NaCl. Samples of the elution fractions (10 × 2.5-ml fractions) were labeled with FP-biotin as described above, and those fractions containing the 75-kDa- and 85-kDa-labeled proteins were pooled and passed over a Mono-Q Sepharose column. Proteins were eluted from the Mono-Q column with a linear gradient of 200–500 mM NaCl, and those elution fractions enriched in the two labeled proteins were then run on SDS/PAGE and transferred to polyvinylidene difluoride (PVDF) membranes by electroblotting. Regions of the PVDF membranes containing the 75- and 85-kDa FP-biotin-reactive proteins were excised, digested with trypsin, and the resulting peptides analyzed by MALDI and MALDI-postsource decay time-of-flight mass spectrometry (15) on a Kratos Kompact Seq Instrument (Kratos Analytical Instruments) equipped with a curved-field reflectron. The MALDI peptide data were used in MS-Fit and MS-Tag searches of the ProteinProspector databases (falcon.ludwig.ucl.ac.uk/mshome3.2.htm), which identified the 75-kDa protein as the rat orthologue of a human protein sequence KIAA0436 and the 85-kDa protein as acylpeptide hydrolase (APH).
Expression of Serine Hydrolases in HEK-293 Cells.
The rat APH cDNA was cloned as follows. Primers were designed on the basis of the enzyme's cDNA sequence (16) and used in PCR experiments to amplify a 1.4-kb partial cDNA clone from a rat liver 5′ Stretch Plus cDNA library (CLONTECH). This amplified cDNA was used as a probe to isolate a full-length APH cDNA from a liver library. The APH cDNA was subcloned into the eukaryotic expression vector, pcDNA3, and transiently transfected into HEK-293 cells by using methods described previously (17). Transfected cells were harvested by trypsinization, washed with Hepes buffer (125 mM Hepes, pH 8.0/100 mM NaCl) and Dounce homogenized in Hepes buffer. Cytosolic and membrane fractions were isolated as described previously (17) and labeled with FP-biotin as detailed above.
Results and Discussion
Design and Synthesis of a Biotinylated Fluorophosphonate, FP-Biotin.
For the generation of a tagged activity-based probe for the serine hydrolase family of enzymes, we considered several possible reactive groups and labeling strategies. Previous work by Glynn and colleagues had demonstrated that a saligenin phosphoramidate was a potent inhibitor of neuropathy target esterase (NTE) and could be synthesized with a biotin tag to identify this protein in tissue extracts (18). However, this inhibitor displayed remarkable specificity for NTE in these experiments and thus appeared too selective to be useful as a general probe for serine hydrolases. Powers and colleagues had generated isocoumarin inhibitors coupled to biotin as serine hydrolase inhibitors (19, 20). Although these isocoumarins reacted with a greater range of serine hydrolases than the aforementioned saligenin phosphoramidate, the requirement that these compounds alkylate a second functional group in the enzyme active site to achieve stable irreversible inhibition suggested that a significant number of serine hydrolases might show poor sensitivity to such reagents. In contrast, FP inhibitors seemed to satisfy the dual requirement of displaying (i) reactivity against the majority of serine hydrolases, and (ii) selectivity for this enzyme family among the various classes of hydrolytic enzymes. Although radiolabeled FPs were available commercially and through our own synthetic efforts (21), the detection of such agents by fluorography requires several days to weeks (21, 22), greatly limiting their general utility as rapid and high-sensitivity probes for profiling serine hydrolase expression and function. Therefore, we devised a route for the chemical synthesis of a biotinylated FP in which the reactive group and the biotin tag were coupled through a linker composed of a long alkyl chain and two amide bonds [FP-biotin (7); see Scheme 1 and Experimental Procedures].
Briefly, 10-undecen-1-ol (1) was converted to iodinated compound 3 through a tosylate intermediate (2). Reaction of 3 with excess triethylphosphite under reflux conditions afforded the diethoxy phosphonate 4, which was converted to the ethoxyhydroxy phosphonate 5 by treatment with trimethylsilylbromide (TMSBr). The double bond of 6 was oxidatively cleaved with ruthenium trichloride and sodium periodate (23) to yield the terminal carboxylic acid product 6. Treatment of 6 with excess diethylaminosulfur trifluoride (DAST) and N-hydoxysuccinimide (NHS) afforded an N-succinyl fluorophosphonate intermediate, which was reacted with 5-(biotinamido) pentylamine (NH2-biotin) to generate FP-biotin (7). This synthetic route also allowed for the facile coupling of 6 to other reporter groups, including fluorescein cadaverine, which generated a fluorescent fluorophosphonate, FP-fluorescein [8; MALDI-FTMS (DHB) m/z 778.2671 (C38H47FN3O8PS + Na+ requires 778.2703)]. FP-fluorescein will be the subject of future investigations aimed at imaging serine protease activities in whole cells.
FP-Biotin Is an Activity-Based Probe for Serine Hydrolases.
To initially test FP-biotin's utility as an activity-based probe for serine hydrolases, we reacted this agent with the mammalian serine amidase, fatty acid amide hydrolase (FAAH) (24). As expected, FP-biotin behaved as a potent irreversible inhibitor of FAAH (data not shown), displaying properties similar to those of other FP inhibitors of the enzyme (21, 25). Recent efforts in our laboratory have shown that serine residue 241 serves as FAAH's catalytic nucleophile, and mutation of this residue to alanine (S241A) generates an inactive enzyme (21). Therefore, FP-biotin (2 μM) was reacted with both FAAH and the S241A mutant (80 nM) for 10 min, after which the proteins were subjected to standard SDS/PAGE-Western blotting procedures by using either anti-FAAH antibodies or avidin as detection reagents (Fig. 1A). Although anti-FAAH antibodies identified both FAAH and the S241A mutant, avidin detected only FAAH in the FP-biotin reactions, demonstrating that this inhibitor exclusively reacted with the active form of the enzyme.
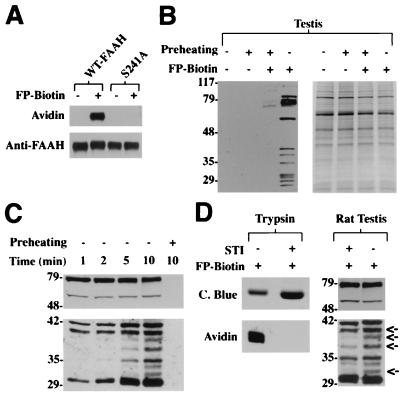
Figure 1.
FP-biotin reacts with serine hydrolases in an activity-dependent manner. (A) Wild-type FAAH or a mutant FAAH, S241A, in which the enzyme's serine nucleophile was mutated to alanine (80 nM protein) was incubated in either the presence or absence of FP-biotin (2 μM) for 10 min, after which protein was separated from excess inhibitor by SDS/PAGE, electroblotted, and detected by using either avidin or anti-FAAH antibodies. (B) Soluble protein from rat testis (1 μg/μL) was treated with FP-biotin (2 μM) either with or without a preheating step (80°C, 5 min), run on SDS/PAGE (10 μg protein/lane), and visualized by blotting with avidin (Left). Coomassie blue staining confirmed that all lanes contained approximately equal amounts of protein (Right). (C) Rates of reactivity of serine hydrolases with FP-biotin. Testis protein (1 μg/μL) was treated with FP-biotin (2 μM) for the indicated times and analyzed as in B. (Top and Bottom) Taken from film exposures of 1 and 8 min, respectively. (D) (Left) Equal amounts of trypsin (2 μM) were preincubated for 2 hr in either the absence or presence of 1.5 molar equivalents of STI, treated with FP-biotin for 30 min, and analyzed as in B. The lower quantity of trypsin observed in the sample without STI (Top, Coomassie blue staining) is likely the result of a moderate degree of self-proteolysis taking place during the preincubation step. (Right) Testis protein (1 μg/μL) was preincubated with 10 μM STI for 20 min, treated with FP-biotin for 10 min, and analyzed as in B. Arrows highlight proteins whose FP-biotin labeling intensities were reduced significantly (at least 2-fold) in the STI-treated sample relative to a control sample. (Top and Bottom) Taken from film exposures of 1 and 8 min, respectively.
To explore further FP-biotin's reactivity with serine hydrolases, we incubated soluble fractions of rat testis with this inhibitor. Consistent with the abundance of proteases found in this tissue (26), FP-biotin labeled more than 10 testicular proteins (Fig. 1B). Phosphonylated proteins of a variety of molecular masses were observed, ranging from 20 to 100 kDa, with a high concentration of labeled proteins found between 25 and 40 kDa, possibly representing members of the kallikrein clan of serine proteases (27). Importantly, heating the protein sample (80°C, 5 min) before treatment with FP-biotin blocked nearly all protein labeling, further supporting that this tagged inhibitor reacts with serine hydrolases in an activity-dependent manner (Fig. 1B). Kinetic analyses revealed that the identified serine hydrolases displayed remarkably different rates of FP-biotin reactivity, with two of the larger proteins labeling to apparent completion within 1 min (Fig. 1C Top) and most of the smaller proteins reacting more slowly over the course of several minutes (Fig. 1C Bottom).
Considering that many serine proteases exist in vivo as inactive complexes with endogenous inhibitory proteins (7, 8, 26), we compared the ability of FP-biotin to react with both free and inhibitor-bound proteases. Although FP-biotin reacted strongly with free trypsin, the tagged inhibitor did not label a trypsin sample that was preincubated with the Kunitz-type serine protease inhibitor, STI, despite the presence in the latter reaction of significantly greater amounts of trypsin (Fig. 1D Left). Soluble fractions of rat testis were also exposed to STI and then treated with FP-biotin. Consistent with the relatively broad specificity of this protease inhibitor, several, but not all, FP-biotin-reactive proteins showed significantly lower labeling intensities in the STI-treated sample (Fig. 1D Right).
Collectively, these results highlight that FP-biotin can detect differences in the functional state of a serine hydrolase, even in the special cases where enzyme activity varies without correlation to enzyme quantity. Such observations gain particular significance when one considers the complexity and diversity of serine proteases and inhibitors typically present in whole-cell and tissue samples. Without an activity-based probe like FP-biotin, standard genomics and/or proteomics studies would have difficulty distinguishing free (active) from inhibitor-bound (inactive) proteases in these samples. Finally, the ability to monitor rates of FP-biotin labeling should greatly assist in the identification of even quite subtle changes in serine hydrolase activities. On this note, we have recently determined the rates of FP-biotin labeling for a panel of FAAH mutants, permitting a quantitative comparison of their respective nucleophile strengths (28).
Molecular Characterization of FP-Biotin-Reactive Proteins.
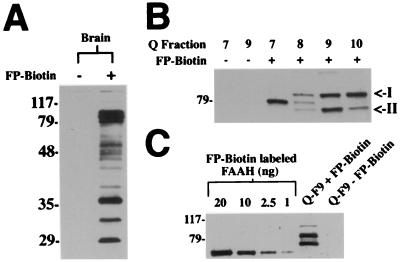
To verify that the proteins labeled by FP-biotin in crude tissue extracts were indeed serine hydrolases, two phosphonylated proteins were isolated from rat brain. The most strongly labeled brain-soluble proteins ranged from 75 to 85 kDa in size (Fig. 2A) and eluted from a Q Sepharose column between 300 and 450 mM NaCl (Fig. 2B). To estimate the abundance of these proteins in the Q elutions, the intensity of their labeling with FP-biotin was compared with that of a serial dilution of a FAAH sample reacted to completion with the inhibitor (Fig. 2C). Both the 75- and 85-kDa FP-biotin-reactive proteins displayed labeling intensities similar to that of a 20-ng sample of FAAH (0.35 pmol), setting a lower limit for the quantity of these proteins that was well within the range needed to obtain protein-sequence information. The 85- and 75-kDa proteins were identified by standard protein chemistry techniques as acylpeptide hydrolase (APH) (16), a serine peptidase that has been shown to react with diisopropyl fluorophosphate (DIFP) (29), and the rat orthologue of a human protein sequence KIAA0436 (10). Interestingly, a homology search revealed that the KIAA0436 protein shares 30% identity with the prokaryotic enzyme Protease II, also an established serine hydrolase that reacts with DIFP (30). The Ser-His-Asp catalytic triad residues of Protease II were conserved in the KIAA0436 protein, supporting that this mammalian protein is a member of the Protease II family of serine proteases. Finally, FP-biotin also labeled a 100-kDa brain protein that appeared to be expressed at much lower levels (equivalent to 15 fmol, or ≈1 ng, of FAAH), demonstrating that this tagged inhibitor can readily detect subnanomolar concentrations of serine hydrolases (15 fmol/20 μl per gel lane).
Figure 2.
Identification of FP-biotin-reactive proteins from rat brain. (A) Soluble fractions of rat brain (1 μg/μL) were incubated in either the presence or absence of FP-biotin (2 μM) for 30 min and then resolved by SDS/PAGE and blotting with avidin. (B) Separation of rat brain proteins by Q-Sepharose chromatography and labeling of the NaCl elution fractions with FP-biotin. Elution fractions 7–10 (300–500 mM NaCl) are shown. Arrows point to the 75-kDa and 85-kDa FP-biotin-reactive proteins for which sequence information was obtained. (C) The signal intensities of FP-biotin-reactive proteins in fraction 9 were compared with those from a titration of known quantities of purified FAAH reacted to completion with FP-biotin.
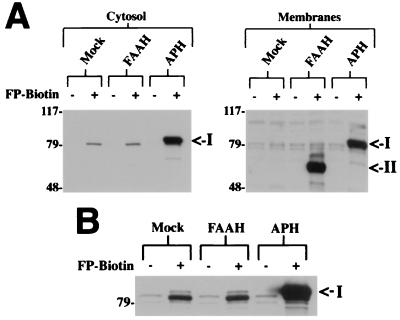
To test whether FP-biotin could record changes in the expression level of serine hydrolases in crude cellular extracts, we transfected cDNAs for both APH and FAAH into HEK-293 cells. Treatment of the cytosolic and membrane fractions of these cells with FP-biotin identified a strongly phosphonylated 85-kDa protein in the APH-transfected cells (Fig. 3 A; I), but not in control cells transfected with either empty vector or the FAAH cDNA. In contrast to this labeling pattern, an abundant 65-kDa phosphonylated protein was identified exclusively in the membrane fraction of FAAH-transfected cells (Fig. 3A; II), consistent with previous characterizations of this serine hydrolase as an integral membrane protein (17, 31). Longer exposures of the cytosol blot identified in the mock and FAAH-transfected HEK cells a weak 85-kDa signal that may represent endogenous levels of APH in this cell type (Fig. 3B; I).
Figure 3.
FP-biotin detects changes in the expression level of serine hydrolases. (A) Protein samples from HEK-293 cells transfected with a FAAH cDNA, APH cDNA, or empty vector (Mock) were reacted with FP-biotin and resolved by SDS/PAGE (10 μg protein/lane) and blotting with avidin. A strongly labeled 85-kDa protein (I) was detected exclusively in the cytosolic and membrane fractions of APH-transfected cells, whereas a strongly labeled 65-kDa protein (II) was observed specifically in the membrane fractions of FAAH-transfected cells. (B) A longer exposure time of the cytosol blot (2 min vs. 10 sec in A) identified an 85-kDa FP-biotin-reactive protein (I) in the mock and FAAH-transfected HEK cells, possibly representing endogenous levels of APH in this cell type.
In summary, the data presented in Figs. 1–3 demonstrate that FP-biotin can: (i) react with numerous serine hydrolases in crude cell and tissue samples, (ii) detect subnanomolar concentrations of serine hydrolases, and (iii) record differences in both the functional state and expression level of these enzymes. It is also important to highlight that the identification of FP-biotin labeled proteins using standard avidin-horseradish peroxidase chemiluminescence assays is extremely rapid (requiring exposure times of only seconds to minutes), making this chemical agent particularly well suited for high-throughput proteomics investigations. Additionally, the covalent attachment of a biotin molecule to phosphonylated serine hydrolases should assist in the subsequent biochemical characterization of these enzymes. For example, Schriemer and colleagues have developed a method that combines immobilized avidin beads with MALDI mass spectrometry to facilitate the chemical analysis of biotinylated proteins and peptides (32). If integrated with FP-biotin, this technique may allow for the molecular identification and functional comparison of serine hydrolases (as well as their respective catalytic nucleophiles) directly from whole-cell and tissue samples.
Profiling Serine Hydrolases in Rat Tissues with FP-Biotin.
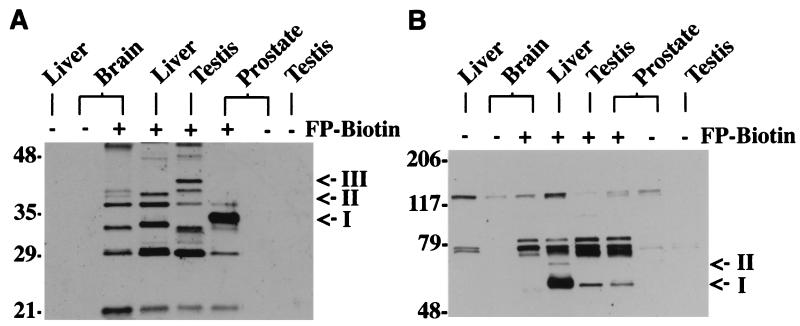
To test FP-biotin's ability to resolve complex patterns of serine hydrolase expression, we compared the profiles of phosphonylated proteins from soluble extracts of rat brain, liver, testis, and prostate (Fig. 4). In the lower molecular-mass range, clear tissue-specific and tissue-restricted FP-biotin-reactive proteins were identified (Fig. 4A). Interestingly, a strongly labeled 33-kDa protein was identified exclusively in prostate (Fig. 4A; I). Although the molecular size of this phosphonylated protein is consistent in mass with human prostate specific antigen (PSA) (33), a serine protease expressed primarily in this tissue, orthologues of PSA are not thought to exist in rodents on the basis of previous molecular (Southern blots) and cell biological (immunocytochemistry) studies (34). The identification of an FP-biotin-reactive protein abundantly and selectively expressed in rat prostate suggests that this organism may indeed possess functional (but not necessarily high sequence-related) homologues of human PSA, an observation that merits further investigation considering PSA's status as a principal marker for prostate cancer (35). Several other FP-biotin-reactive proteins also displayed tissue-restricted patterns of expression, including a testis-specific 42-kDa protein (Fig. 4A; III) and two 38–40 kDa proteins, one of which was found in brain and testis and the other in brain and liver (Fig. 4A; II). In the larger molecular-mass range, most of the FP-biotin-reactive proteins appeared to display broad tissue distributions (Fig. 4B). However, a labeled 65-kDa protein was found in highest relative abundance in liver, at lower levels in testis and prostate, and was not detected in brain (Fig. 4B; I). Similarly, a phosphonylated 70-kDa protein was found exclusively in liver (Fig. 4B; II), even on longer exposures (data not shown).
Figure 4.
FP-biotin identifies several candidate serine hydrolase activities with tissue-restricted patterns of expression. (A) Soluble fractions from indicated rat tissues (1 μg/μL) were treated with FP-biotin (2 μM) and resolved by SDS/PAGE (10 μg protein/lane; 14% polyacrylamide gel) and blotting with avidin. Arrows point to phosphonylated proteins specifically expressed in prostate (I), testis (III), and brain/liver or brain/testis (II). (B) Same as in A, except samples were run on an 8% polyacrylamide gel to more clearly resolve FP-biotin-reactive proteins of higher molecular mass. Arrows point to proteins expressed predominantly (I) or exclusively (II) in liver. Weakly avidin-reactive proteins in the samples untreated with FP-biotin represent putative endogenously biotinylated proteins (18).
Conclusions
Herein, we describe a powerful method for monitoring dynamics in the expression and function of an entire enzyme family. Although we have demonstrated the utility of a biotinylated fluorophosphonate as a rapid and high-sensitivity probe for detecting serine hydrolase activities directly from crude cell and tissue samples, one could envision that additional types of tagged irreversible inhibitors may succeed at labeling other classes of enzymes. For example, Bogyo and colleagues have recently used radiolabeled vinyl sulfones as selective reagents for marking members of the proteasome family of proteases (36). Although tagged irreversible inhibitors should prove useful in the immediate future for isolating and identifying novel members of large enzyme families, their more enduring purpose in the postgenome era will likely be as class-selective probes for proteomics studies (37–40) aimed at characterizing the role that these proteins play in physiological and/or pathological events. In such experiments, tagged irreversible inhibitors offer the special opportunity to profile proteins on the basis of activity rather than quantity and, through doing so, should record changes in the functional state of an enzyme even in the cases where its levels remain constant.
Acknowledgments
We thank Drs. R. Lerner, N. Gilula, P. Schimmel, C.-H. Wong, J. Kelly, S. Licht, and M. Bracey for critical reading of the manuscript and helpful discussions. Protein digestion and mass spectrometry analysis were done by Dr. J. Leszyk at the University of Massachusetts Medical School Core Laboratory for Protein Microsequencing and Mass Spectrometry (Shrewsbury, MA). This work was supported by grants from the Searle Scholars Program (to B.F.C.), the National Science Foundation (to M.P.P.), and the Skaggs Institute for Chemical Biology.
Abbreviations
- APH
acylpeptide hydrolase
- FAAH
fatty acid amide hydrolase
- FP
fluorophosphonate/fluorophosphate
- MALDI
matrix-assisted laser desorption ionization
- STI
soybean trypsin inhibitor
- equiv
equivalent
- FTMS
Fourier transform MS
- DHB
2,5-dihydroxybenzoic acid
- APH
acylpeptide hydrolase
References
- 1.Kalafatis M, Egan J O, van't Veer C, Cawthern K M, Mann K G. Crit Rev Eukaryotic Gene Expression. 1997;7:241–280. doi: 10.1615/critreveukargeneexpr.v7.i3.40. [DOI] [PubMed] [Google Scholar]
- 2.Clark J D, Schievella A R, Nalefski E A, Lin L L. J Lipid Mediat Cell Signal. 1995;12:83–117. doi: 10.1016/0929-7855(95)00012-f. [DOI] [PubMed] [Google Scholar]
- 3.Mignatti P, Rifkin D B. Enzyme Protein. 1996;49:117–137. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida S, Shiosaka S. Int J Mol Med. 1999;3:405–409. doi: 10.3892/ijmm.3.4.405. [DOI] [PubMed] [Google Scholar]
- 5.Seidah N G, Chretien M. Curr Opin Biotechnol. 1997;8:602–607. doi: 10.1016/s0958-1669(97)80036-5. [DOI] [PubMed] [Google Scholar]
- 6.Smyth M J, O'Conner M D, Trapani J A. J Leukocyte Biol. 1996;60:555–562. doi: 10.1002/jlb.60.5.555. [DOI] [PubMed] [Google Scholar]
- 7.Kato G J. Hum Mutat. 1999;13:87–98. doi: 10.1002/(SICI)1098-1004(1999)13:2<87::AID-HUMU1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Declerck Y A, Imren S, Montgomery A M, Mueller B M, Reisfeld R A, Laug W E. Adv Exp Med Biol. 1997;425:89–97. doi: 10.1007/978-1-4615-5391-5_9. [DOI] [PubMed] [Google Scholar]
- 9.Davies B J, Pickard B S, Steel M, Morris R G, Lathe R. J Biol Chem. 1998;273:23004–23011. doi: 10.1074/jbc.273.36.23004. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa K, Nagase T, Nakajima D, Seki N, Ohira M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O. DNA Res. 1997;4:307–313. doi: 10.1093/dnares/4.5.307. [DOI] [PubMed] [Google Scholar]
- 11.Nelson P S, Gan L, Ferguson C, Moss P, Gelinas R, Hood L, Wang K. Proc Natl Acad Sci USA. 1999;96:3114–3119. doi: 10.1073/pnas.96.6.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creighton T E. Proteins: Structure and Molecular Properties. 2nd Ed. New York: Freeman; 1993. [Google Scholar]
- 13.Walsh C T. Enzymatic Reaction Mechanisms. New York: Freeman; 1979. [Google Scholar]
- 14.Pineiro-Sanchez M L, Goldstein L A, Dodt J, Howard L, Yeh Y, Chen W T. J Biol Chem. 1997;272:7595–7601. doi: 10.1074/jbc.272.12.7595. [DOI] [PubMed] [Google Scholar]
- 15.Chaurand P, Luetzenkirchen F, Spengler B. J Am Soc Mass Spectrom. 1999;10:91–103. doi: 10.1016/S1044-0305(98)00145-7. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi K, Lin L-W, Yeadon J E, Klickstein L B, Smith J A. J Biol Chem. 1989;264:8892–8899. [PubMed] [Google Scholar]
- 17.Giang D K, Cravatt B F. Proc Natl Acad Sci USA. 1997;94:2238–2242. doi: 10.1073/pnas.94.6.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glynn P, Read D J, Guo R, Wylie S, Johnson M K. Biochem J. 1994;301:551–556. doi: 10.1042/bj3010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kam C-M, Abuelyaman A S, Li Z, Hudig D, Powers J C. Bioconjugate Chem. 1993;4:560–567. doi: 10.1021/bc00024a021. [DOI] [PubMed] [Google Scholar]
- 20.Winkler U, Allison N J, Woodard S L, Gault R A, Ewoldt G R, Kam C M, Abuelyaman A, Powers J C. Mol Immunol. 1996;33:615–623. doi: 10.1016/0161-5890(96)00025-9. [DOI] [PubMed] [Google Scholar]
- 21.Patricelli M P, Lovato M A, Cravatt B F. Biochemistry. 1999;38:9804–9812. doi: 10.1021/bi990637z. [DOI] [PubMed] [Google Scholar]
- 22.Keshavarz-Shokri A, Suntornwat O, Kitos P A. Anal Biochem. 1999;267:406–411. doi: 10.1006/abio.1998.3031. [DOI] [PubMed] [Google Scholar]
- 23.Carlsen P H J, Katsuki T, Martin V S, Sharpless K B. J Org Chem. 1981;46:3936–3938. [Google Scholar]
- 24.Cravatt B F, Giang D K, Mayfield S P, Boger D L, Lerner R A, Gilula N B. Nature (London) 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 25.Deutsch D G, Omeir R, Arreaza G, Salehani D, Prestwich G D, Huang Z, Howlett A. Biochem Pharmacol. 1997;53:255–260. doi: 10.1016/s0006-2952(96)00830-1. [DOI] [PubMed] [Google Scholar]
- 26.Monsees T K, Schill W B, Miska W. Adv Exp Med Biol. 1997;424:111–123. doi: 10.1007/978-1-4615-5913-9_20. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald R J, Southard-Smith E M, Kroon E. J Biol Chem. 1996;271:13684–13690. doi: 10.1074/jbc.271.23.13684. [DOI] [PubMed] [Google Scholar]
- 28.Patricelli M P, Cravatt B F. Biochemistry. 1999;38:14125–14130. doi: 10.1021/bi991876p. [DOI] [PubMed] [Google Scholar]
- 29.Scaloni A, Jones W M, Barra D, Pospischil M, Sassa S, Popowicz A, Manning L R, Schneewind O, Manning J M. J Biol Chem. 1992;267:3811–3818. [PubMed] [Google Scholar]
- 30.Yoshimoto T, Tabiri J, Kabashima T, Inoue S, Ito K. J Biochem. 1995;117:654–660. doi: 10.1093/oxfordjournals.jbchem.a124759. [DOI] [PubMed] [Google Scholar]
- 31.Patricelli M P, Lashuel H A, Giang D K, Kelly J W, Cravatt B F. Biochemistry. 1998;37:15177–15187. doi: 10.1021/bi981733n. [DOI] [PubMed] [Google Scholar]
- 32.Schriemer D C, Yalcin T, Li L. Anal Chem. 1998;70:1569–1575. doi: 10.1021/ac9710341. [DOI] [PubMed] [Google Scholar]
- 33.Bei R, Paranavitana C, Milenic D, Kashmiri S V, Schlom J. J Clin Lab Anal. 1995;9:261–268. doi: 10.1002/jcla.1860090408. [DOI] [PubMed] [Google Scholar]
- 34.Karr J F, Kantor J A, Hand P H, Eggensperger D L, Schlom J. Cancer Res. 1995;55:2455–2462. [PubMed] [Google Scholar]
- 35.Polascik T J, Oesterling J E, Partin A W. J Urol. 1999;162:293–306. doi: 10.1016/s0022-5347(05)68543-6. [DOI] [PubMed] [Google Scholar]
- 36.Bogyo M, McMaster J S, Gaczynska M, Tortorella D, Goldberg A L, Ploegh H. Proc Natl Acad Sci USA. 1997;94:6629–6634. doi: 10.1073/pnas.94.13.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkins M R, Williams K L, Appel R, Hochstrasser D F. Proteome Research: New Frontiers in Functional Genomics. Berlin: Springer; 1997. [Google Scholar]
- 38.James P. Q Rev Biophys. 1997;30:279–331. doi: 10.1017/s0033583597003399. [DOI] [PubMed] [Google Scholar]
- 39.Dove A. Nat Biotechnol. 1999;17:233–236. doi: 10.1038/6972. [DOI] [PubMed] [Google Scholar]
- 40.Anderson N L, Anderson N G. Electrophoresis. 1998;19:1853–1861. doi: 10.1002/elps.1150191103. [DOI] [PubMed] [Google Scholar]