Abstract
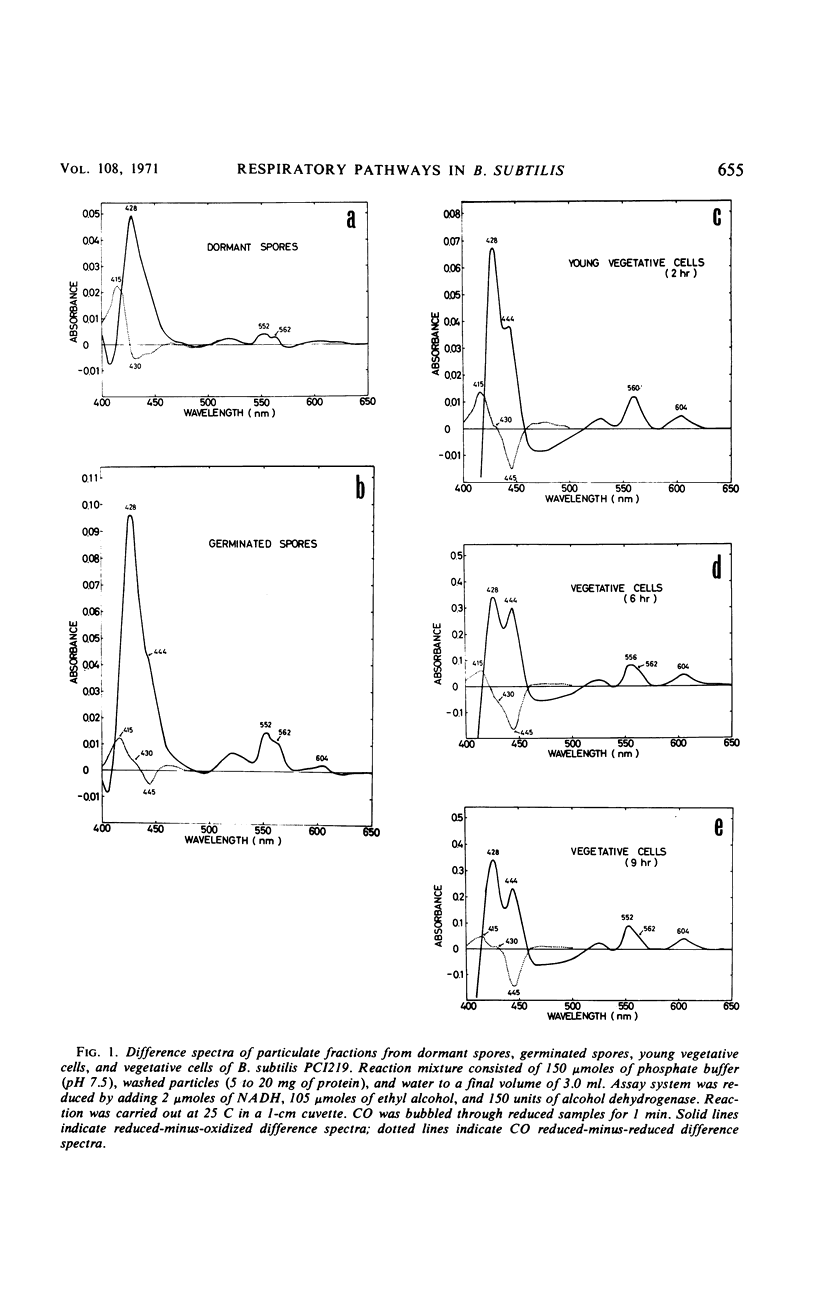
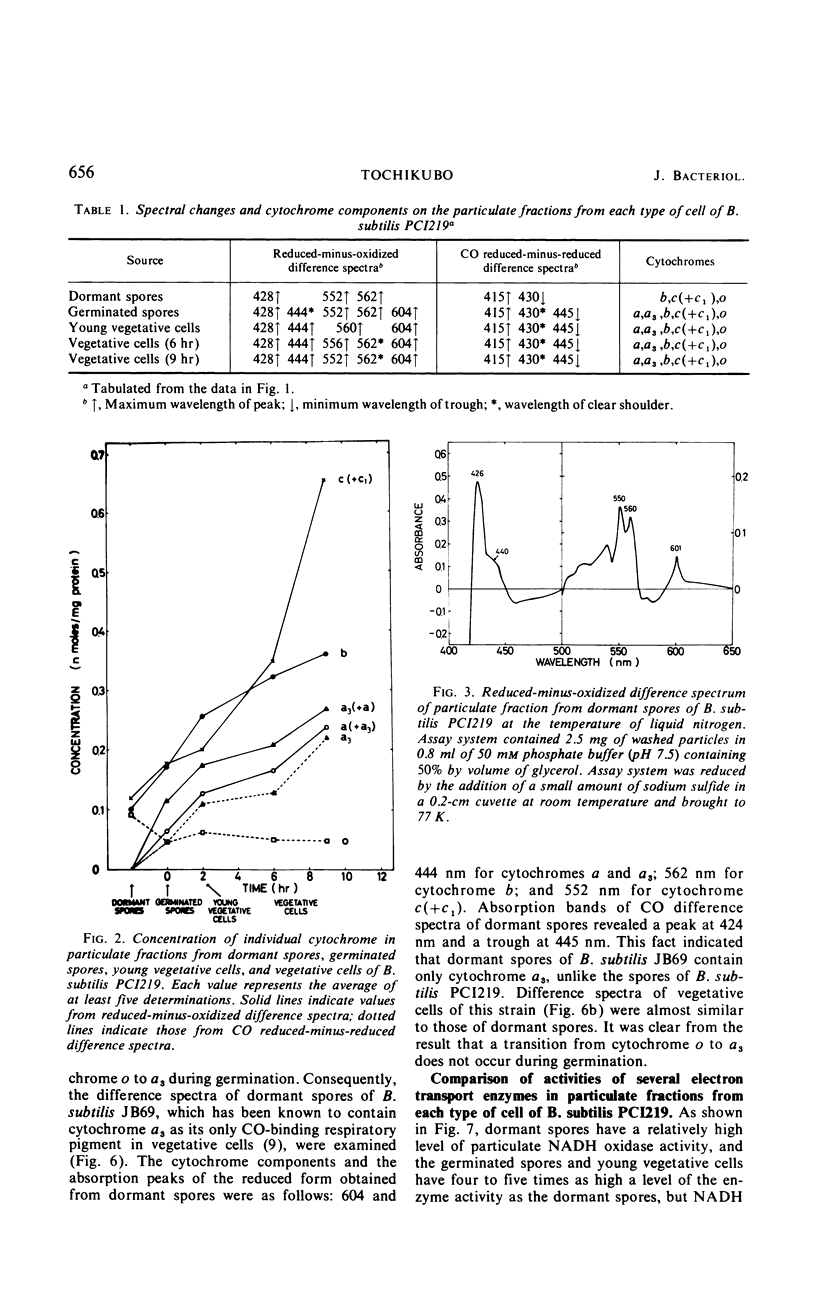
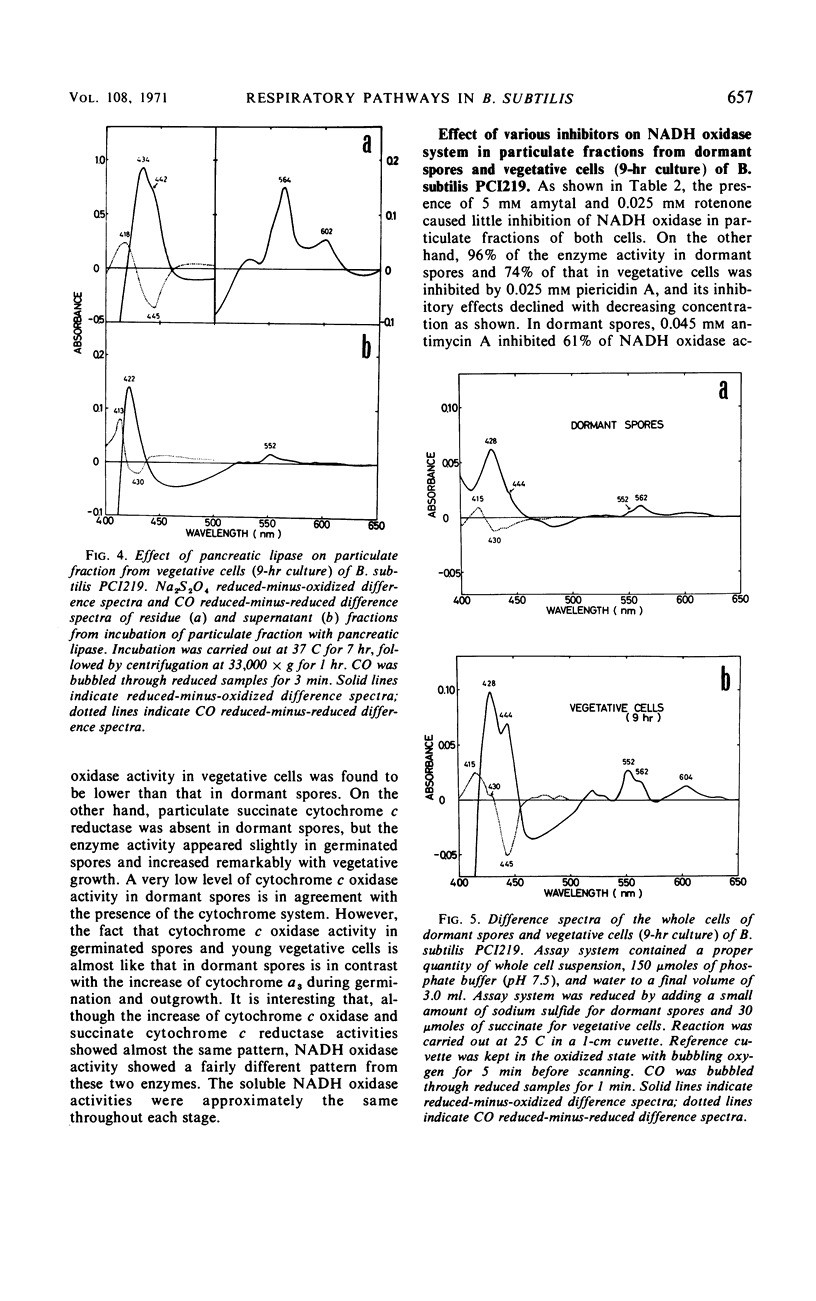
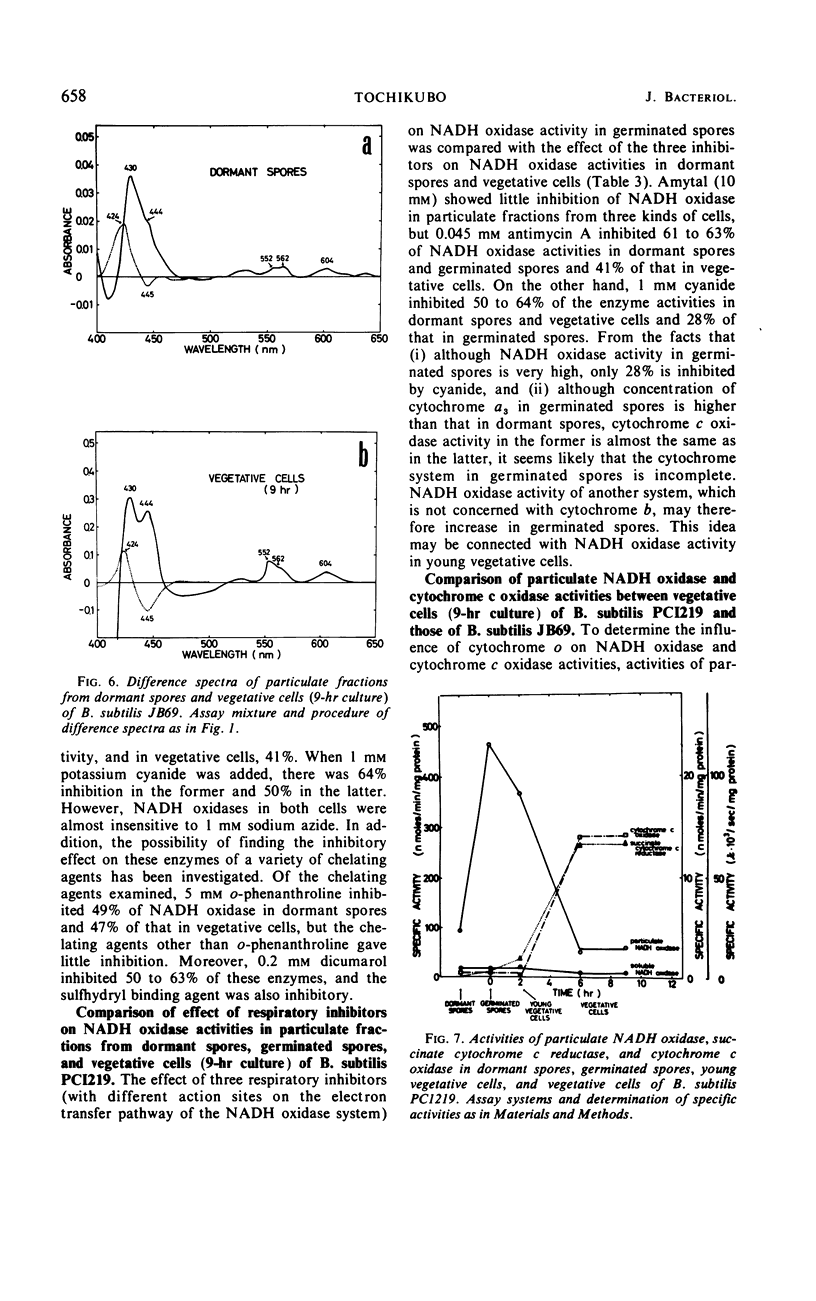
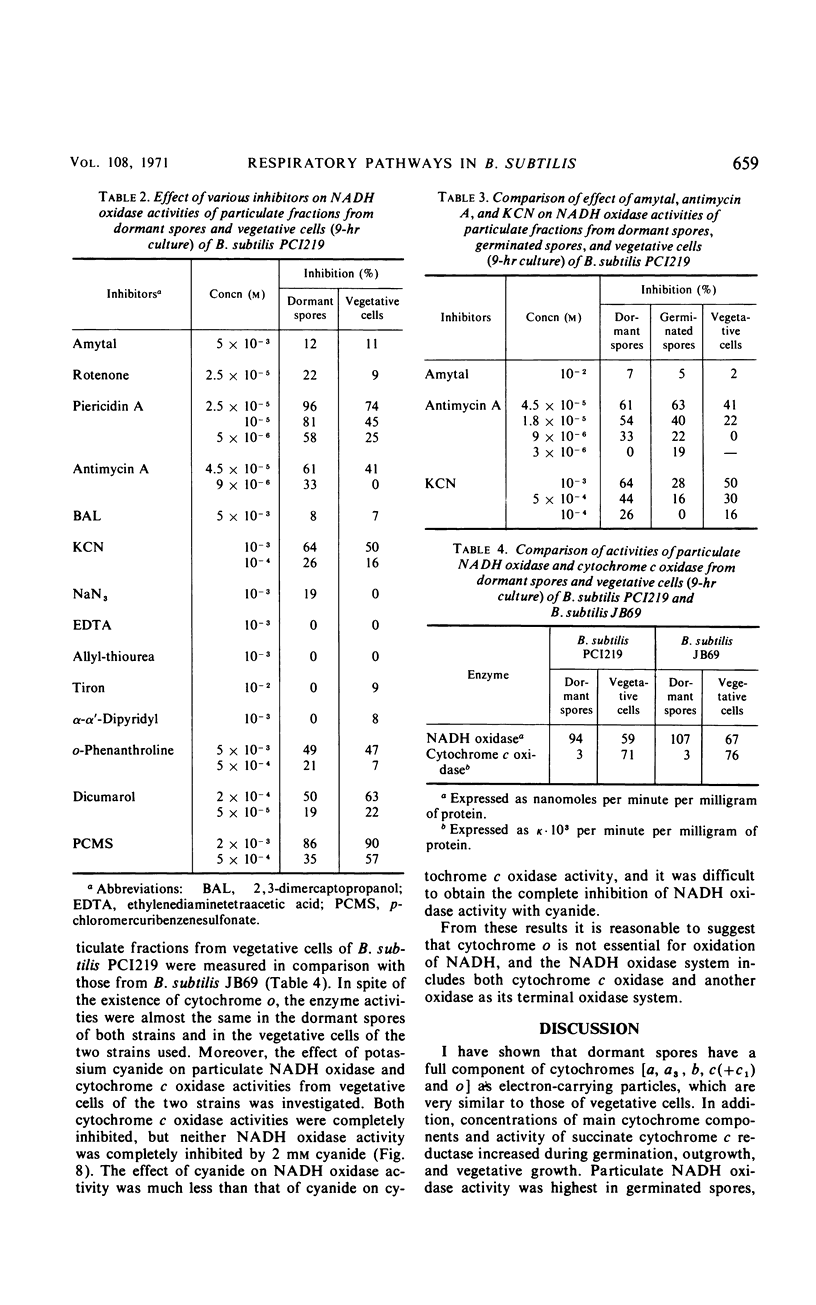
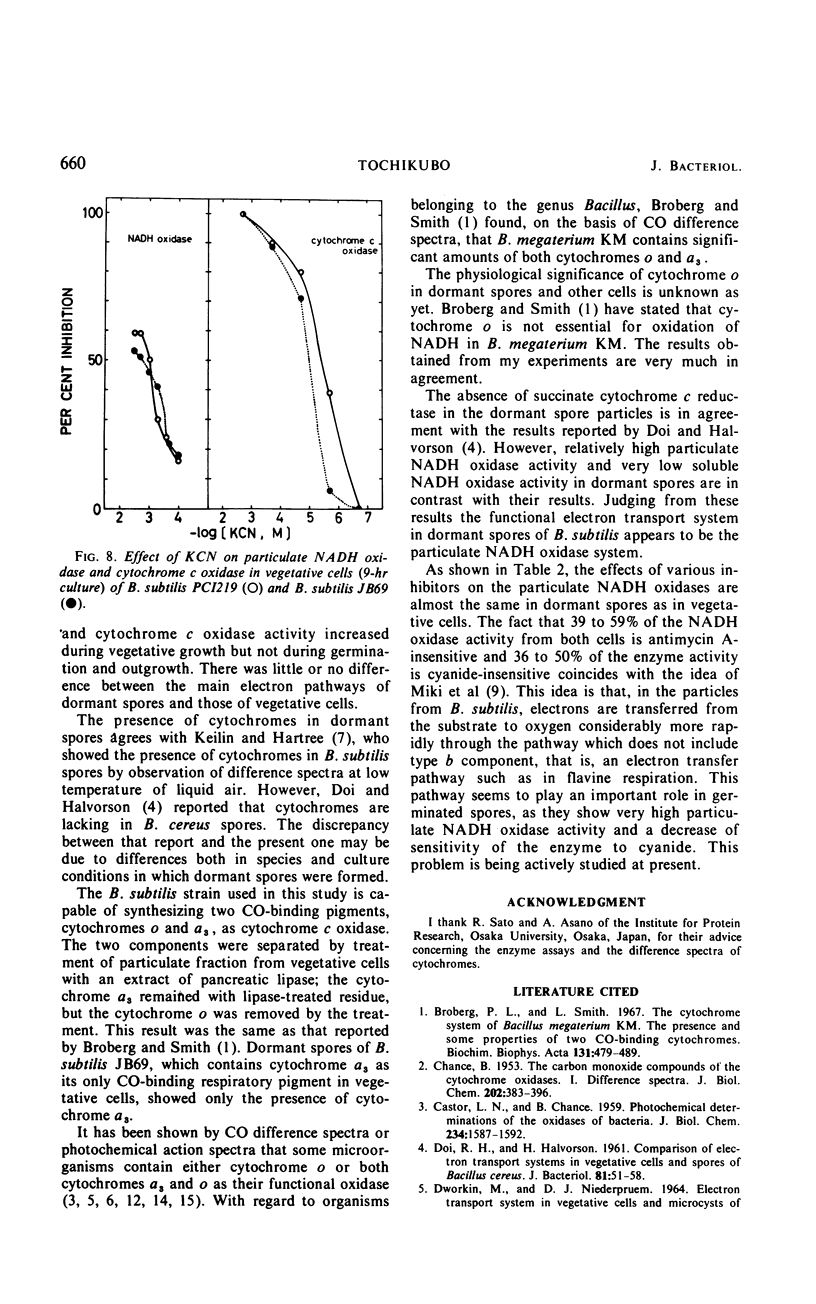
The chemical and enzymatic properties of the cytochrome system in the particulate preparations obtained from dormant spores, germinated spores, young vegetative cells, and vegetative cells of Bacillus subtilis PCI219 were investigated. Difference spectra of particulate fractions from dormant spores of this strain suggested the presence of cytochromes a, a3, b, c(+c1), and o. All of the cytochrome components were present in dormant spores and in germinated spores and vegetative cells at all stages which were investigated. Concentrations of cytochromes a, a3, b, and c(+c1) increased during germination, outgrowth, and vegetative growth, but that of cytochrome o was highest in dormant spores. As the cytochrome components were reducible by reduced nicotinamide adenine dinucleotide (NADH), they were believed to be metabolically active. Difference spectra of whole-cell suspensions of dormant spores and vegetative cells were coincident with those of the particulate fractions. NADH oxidase and cytochrome c oxidase were present in dormant spores, germinated spores, and vegetative cells at all stages after germination, but succinate cytochrome c reductase was not present in dormant spores. Cytochrome c oxidase and succinate cytochrome c reductase activities increased with growth, but NADH oxidase activity was highest in germinated spores and lowest in vegetative cells. There was no striking difference between the effects of respiratory inhibitors on NADH oxidase in dormant spores and those on NADH oxidase in vegetative cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical determinations of the oxidases of bacteria. J Biol Chem. 1959 Jun;234(6):1587–1592. [PubMed] [Google Scholar]
- CHANCE B. The carbon monoxide compounds of the cytochrome oxidases. I. Difference spectra. J Biol Chem. 1953 May;202(1):383–396. [PubMed] [Google Scholar]
- CONRAD H., SMITH L. A study of the kinetics of the oxidation of cytochrome c by cytochrome c oxidase. Arch Biochem Biophys. 1956 Aug;63(2):403–413. doi: 10.1016/0003-9861(56)90055-8. [DOI] [PubMed] [Google Scholar]
- DOI R. H., HALVORSON H. Comparison of electron transport systems in vegetative cells and spores of Bacillus cereus. J Bacteriol. 1961 Jan;81:51–58. doi: 10.1128/jb.81.1.51-58.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DWORKIN M., NIEDERPRUEM D. J. ELECTRON TRANSPORT SYSTEM IN VEGETATIVE CELLS AND MICROCYSTS OF MYXOCOCCUS XANTHUS. J Bacteriol. 1964 Feb;87:316–322. doi: 10.1128/jb.87.2.316-322.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS N. J., CONTI S. F. EFFECT OF HEMIN ON THE FORMATION OF THE CYTOCHROME SYSTEM OF ANAEROBICALLY GROWN STAPHYLOCOCCUS EPIDERMIDIS. J Bacteriol. 1965 Mar;89:675–679. doi: 10.1128/jb.89.3.675-679.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- SCHAEFFER P. Recherches sur le métabolisme bactérien des cytochromes et des porphyrines. I. Disparition partielle des cytochromes par culture anaérobie chez certaines bactéries aérobies facultatives. Biochim Biophys Acta. 1952 Sep;9(3):261–270. doi: 10.1016/0006-3002(52)90160-1. [DOI] [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes; difference spectra. Arch Biochem Biophys. 1954 Jun;50(2):299–314. doi: 10.1016/0003-9861(54)90045-4. [DOI] [PubMed] [Google Scholar]
- SMITH L. Reactions of cytochromes a and a3. II. Studies with Micrococcus pyogenes var. albus and Bacillus subtilis. J Biol Chem. 1955 Aug;215(2):847–857. [PubMed] [Google Scholar]
- TABER H. W., MORRISON M. ELECTRON TRANSPORT IN STAPHYLOCOCCI. PROPERTIES OF A PARTICLE PREPARATION FROM EXPONENTIAL PHASE STAPHYLOCOCCUS AUREUS. Arch Biochem Biophys. 1964 May;105:367–379. doi: 10.1016/0003-9861(64)90021-9. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI S., KAMEN M. D. THE OXIDASE SYSTEM OF HETEROTROPHICALLY-GROWN RHODOSPIRILLUM RUBRUM. Biochim Biophys Acta. 1965 Mar 22;96:395–428. doi: 10.1016/0005-2787(65)90560-5. [DOI] [PubMed] [Google Scholar]
- WEIBULL C., BERGSTROM L. The chemical nature of the cytoplasmic membrane and cell wall of Bacillus megaterium, strain M. Biochim Biophys Acta. 1958 Nov;30(2):340–351. doi: 10.1016/0006-3002(58)90059-3. [DOI] [PubMed] [Google Scholar]



