Abstract
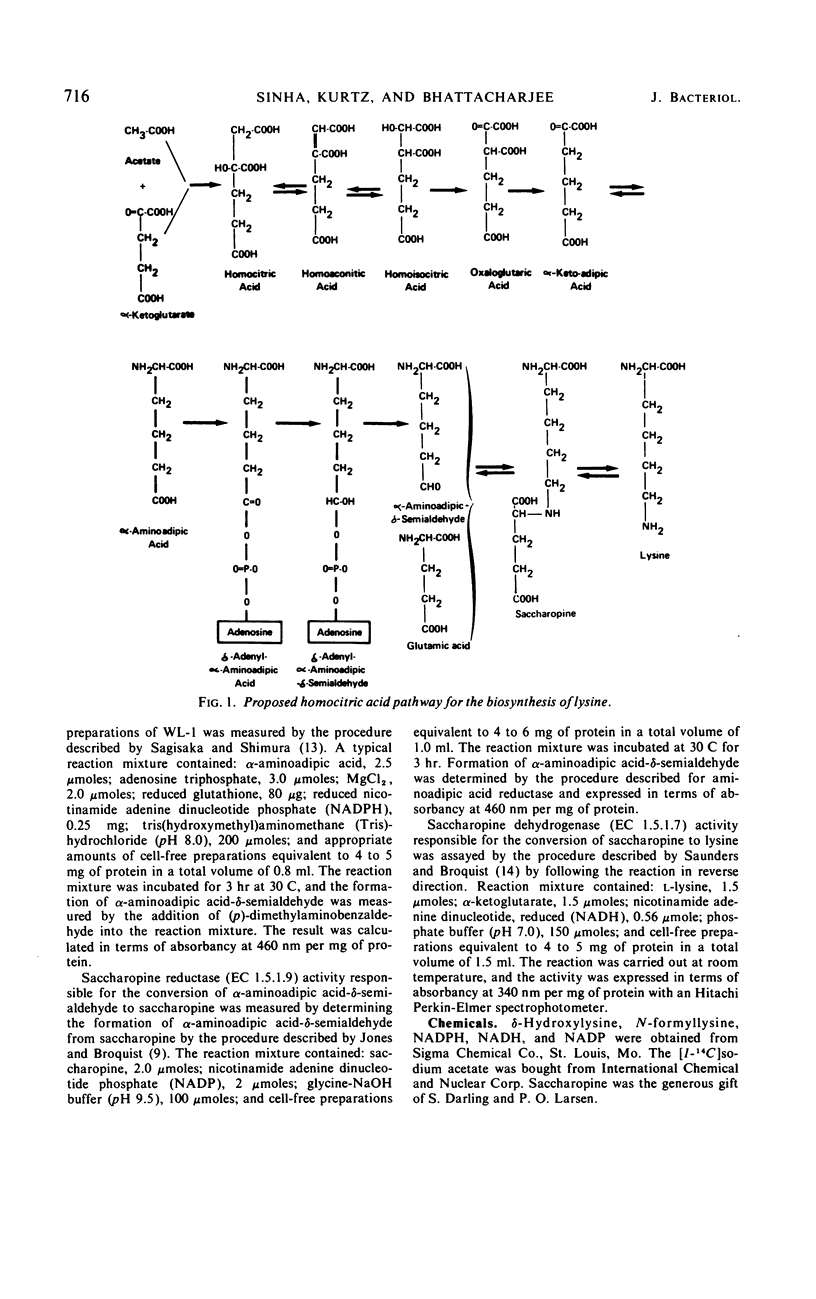
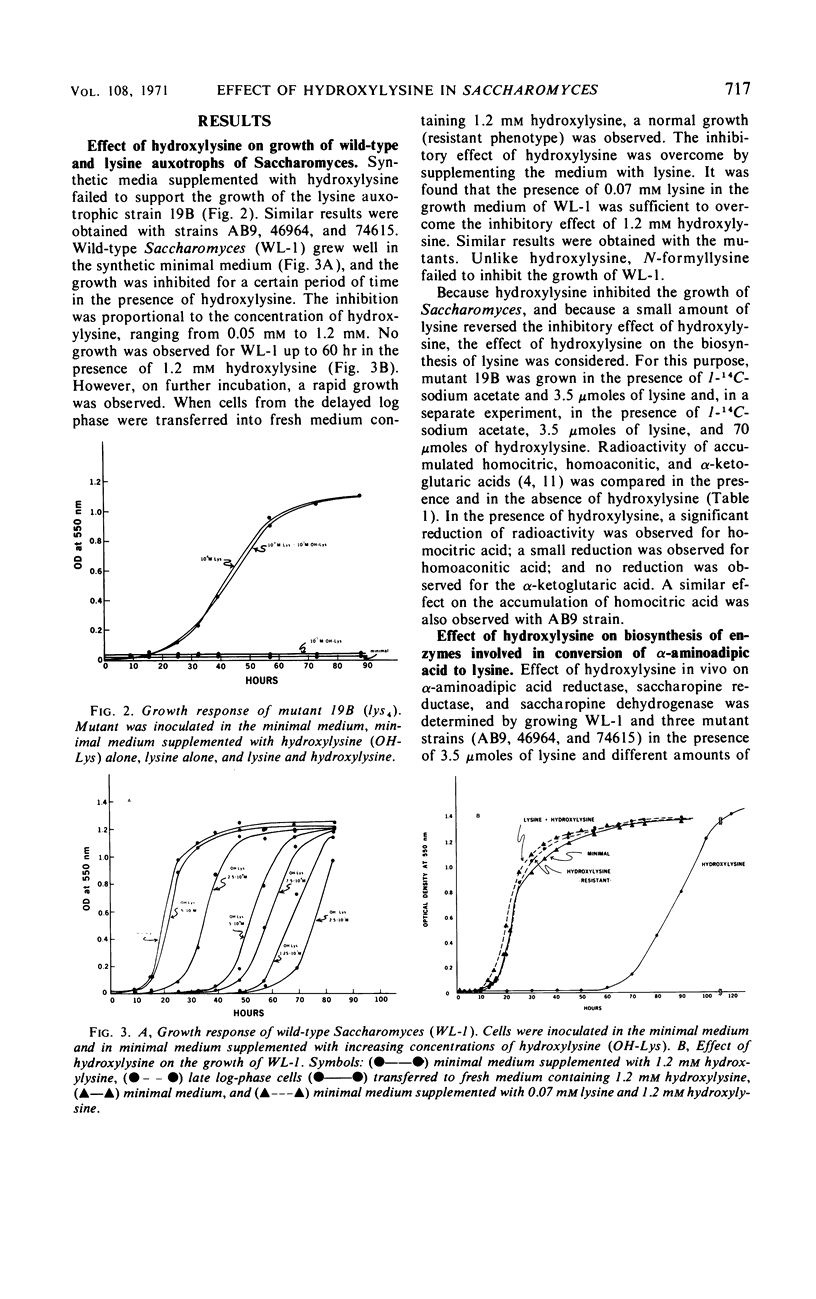
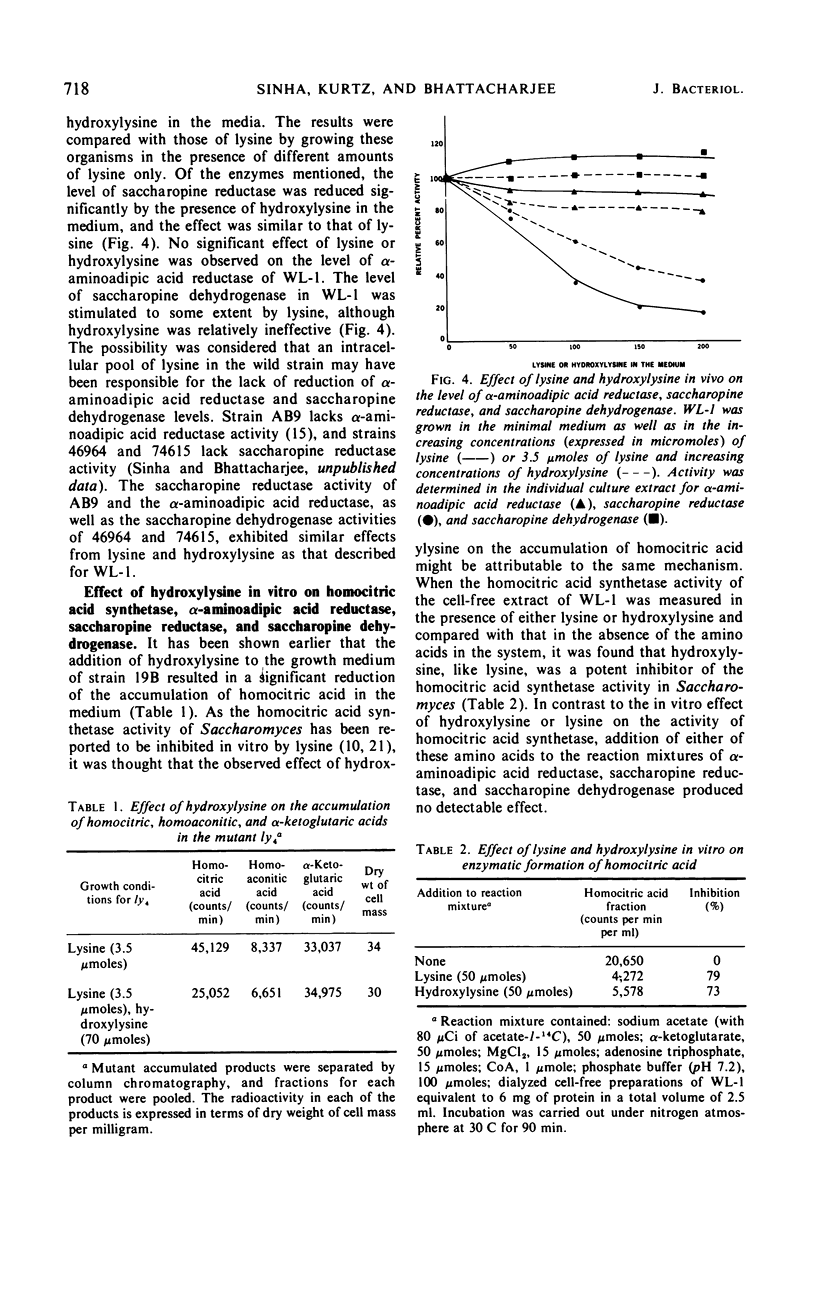
Hydroxylysine acts as a growth inhibitor of Saccharomyces for a certain period of time. The inhibition is concentration-dependent and is reversed by a small amount of lysine in the medium. After the growth-inhibitory period, the wild-type cells are able to grow rapidly even in the presence of hydroxylysine. Both lysine auxotrophs and wild-type cells are unable to utilize hydroxylysine in place of lysine. Hydroxylysine, mimicking lysine, controls the biosynthesis of lysine and thereby limits the availability of biosynthetic lysine to the cells. Hydroxylysine affects the biosynthesis of lysine at a number of enzymatic steps. Accumulation of homocitric acid, the first intermediate of lysine biosynthesis, in the mutant strains 19B and A B9 is reduced significantly in the presence of hydroxylysine. Hydroxylysine, like lysine, exerts a significant inhibition in vitro on the homocitric acid-synthesizing activity. Enzymes following the α-aminoadipic acid step respond in a noncoordinate fashion to hydroxylysine. Level of the enzyme saccharopine reductase, but not of α-aminoadipic acid reductase or saccharopine dehydrogenase, is reduced significantly. These regulatory effects of hydroxylysine are similar to those observed for lysine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhattacharjee J. K. Leaky mutation and coordinate regulation of the accumulation of lysine-precursors in Saccharomyces. Can J Genet Cytol. 1970 Dec;12(4):785–789. doi: 10.1139/g70-102. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee J. K., Strassman M. Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant. J Biol Chem. 1967 May 25;242(10):2542–2546. [PubMed] [Google Scholar]
- Bhattacharjee J. K., Tucci A. F. Relationship of glutaric acid to the homocitric acid pathway of biosynthesis of lysine in yeast. J Biol Chem. 1969 Mar 25;244(6):1417–1423. [PubMed] [Google Scholar]
- Bhattacharjee J. K., Tucci A. F., Strassman M. Accumulation of alpha-ketoglutaric acid in yeast mutants requiring lysine. Arch Biochem Biophys. 1968 Feb;123(2):235–239. doi: 10.1016/0003-9861(68)90129-x. [DOI] [PubMed] [Google Scholar]
- Gilboe D. P., Friede J. D., Henderson L. M. Effect of hydroxylysine on the biosynthesis of lysine in Streptococcus faecalis. J Bacteriol. 1968 Mar;95(3):856–863. doi: 10.1128/jb.95.3.856-863.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass J., Bhattacharjee J. K. Biosynthesis of lysine in Rhodotorula: accumulation of homocitric, homoaconitic, and homoisocitric acids in a leaky mutant. Genetics. 1971 Mar;67(3):365–376. doi: 10.1093/genetics/67.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg R. W., Broquist H. P. Homocitrate formation in Neurospora crassa. Relation to lysine biosynthesis. J Biol Chem. 1968 Apr 25;243(8):1839–1845. [PubMed] [Google Scholar]
- Jones E. E., Broquist H. P. Saccharopine, an intermediate of the aminoadipic acid pathway of lysine biosynthesis. 3. Aminoadipic semialdehyde-glutamate reductase. J Biol Chem. 1966 Jul 25;241(14):3430–3434. [PubMed] [Google Scholar]
- Maragoudakis M. E., Holmes H., Strassman M. Control of lysine biosynthesis in yeast by a feedback mechanism. J Bacteriol. 1967 May;93(5):1677–1680. doi: 10.1128/jb.93.5.1677-1680.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragoudakis M. E., Strassman M. Homocitric acid accumulation by a lysine-requiring yeast mutant. J Biol Chem. 1966 Feb 10;241(3):695–699. [PubMed] [Google Scholar]
- SAGISAKA S., SHIMURA K. Enzymic reduction of alpha-amino-adipic acid by yeast enzyme. Nature. 1959 Nov 28;184(Suppl 22):1709–1710. doi: 10.1038/1841709b0. [DOI] [PubMed] [Google Scholar]
- Saunders P. P., Broquist H. P. Saccharopine, an intermediate of the aminoadipic acid pathway of lysine biosynthesis. IV. Saccharopine dehydrogenase. J Biol Chem. 1966 Jul 25;241(14):3435–3440. [PubMed] [Google Scholar]
- Sinha A. K., Bhattacharjee J. K. Control of a lysine-biosynthetic step by two unlinked genes of Saccharomyces. Biochem Biophys Res Commun. 1970;39(6):1205–1210. doi: 10.1016/0006-291x(70)90689-3. [DOI] [PubMed] [Google Scholar]
- Strassman M., Ceci L. N. Enzymatic formation of alpha-ketoadipic acid from homoisocitric acid. J Biol Chem. 1965 Nov;240(11):4357–4361. [PubMed] [Google Scholar]
- Strassman M., Ceci L. N. Enzymatic formation of cis-homoaconitic acid, an intermediate in lysine biosynthesis in yeast. J Biol Chem. 1966 Nov 25;241(22):5401–5407. [PubMed] [Google Scholar]
- Strassman M., Ceci L. N. Enzymatic formation of homocitric acid, an intermediate in lysine biosynthesis. Biochem Biophys Res Commun. 1964;14:262–267. doi: 10.1016/0006-291x(64)90446-2. [DOI] [PubMed] [Google Scholar]
- Truffa-Bachi P., Cohen G. N. Some aspects of amino acid biosynthesis in microorganisms. Annu Rev Biochem. 1968;37:79–108. doi: 10.1146/annurev.bi.37.070168.000455. [DOI] [PubMed] [Google Scholar]
- Tucci A. F. Feedback inhibition of lysine biosynthesis in yeast. J Bacteriol. 1969 Aug;99(2):624–625. doi: 10.1128/jb.99.2.624-625.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


