Abstract
Three types of evidence are presented to show that the enzymes that hydroxylate nicotinic acid to 2,6-dihydroxynicotinic acid use water as a source of oxygen atoms. 18O is incorporated into the products from H218O. Molecular oxygen acts as a terminal electron acceptor, one-half molecule being consumed per molecule of hydroxyl groups incorporated. An external electron acceptor is required for activity in purified preparations.
Full text
PDF
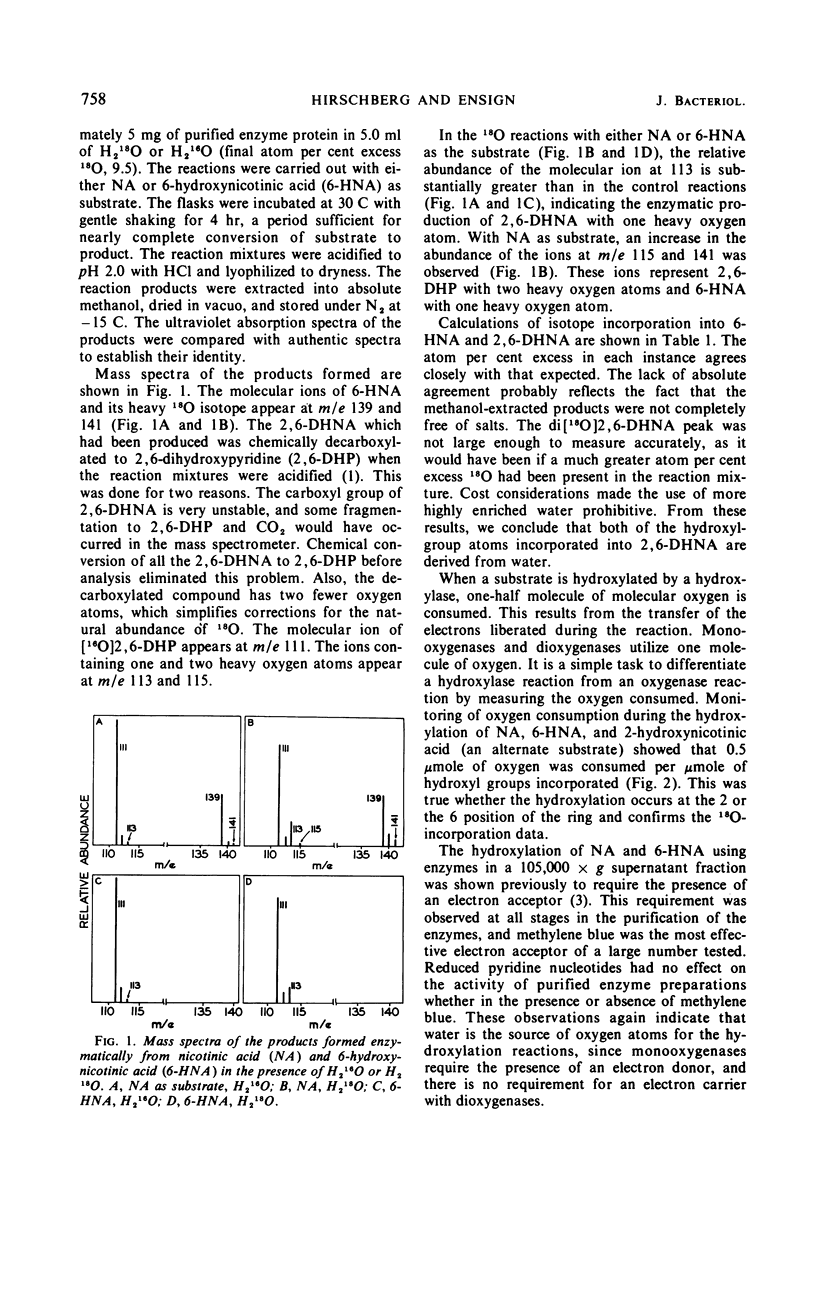
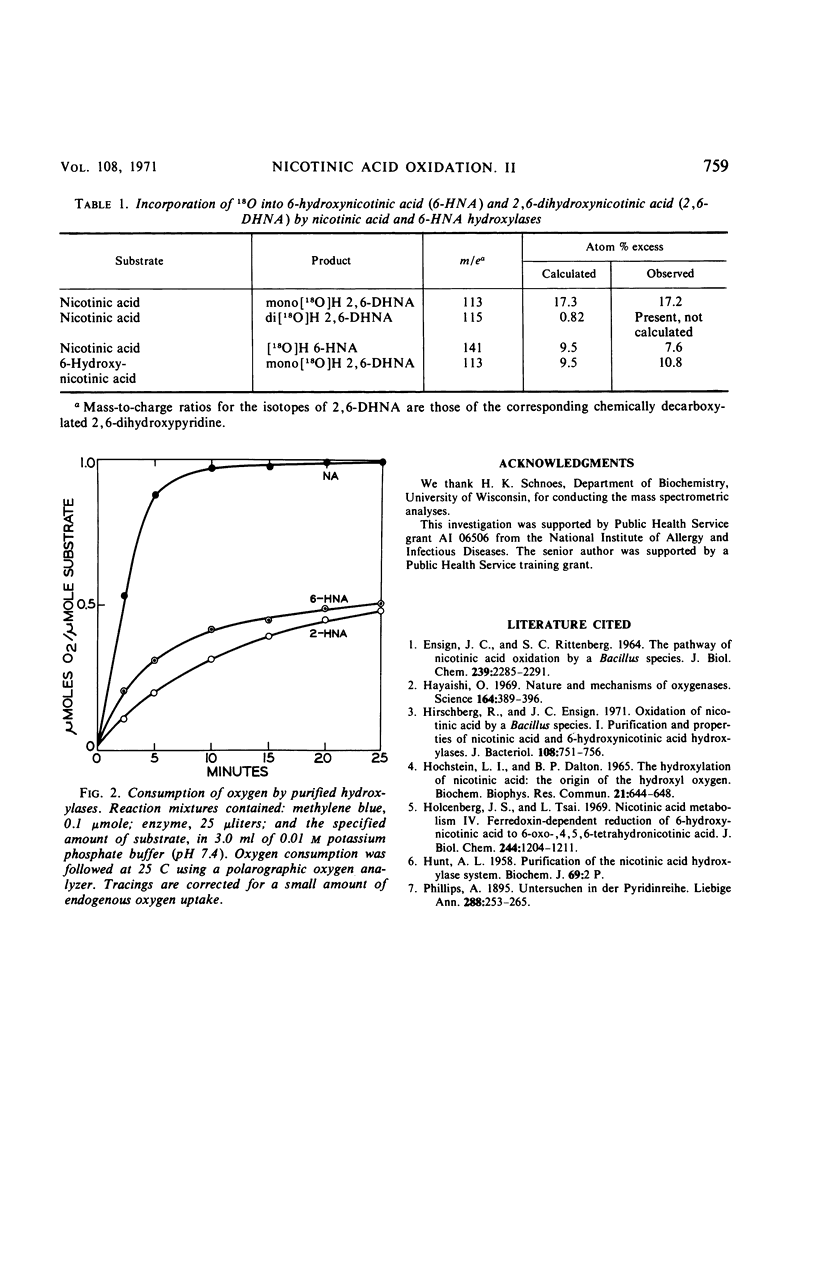
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ENSIGN J. C., RITTENBERG S. C. THE PATHWAY OF NICOTINIC ACID OXIDATION BY A BACILLUS SPECIES. J Biol Chem. 1964 Jul;239:2285–2291. [PubMed] [Google Scholar]
- Hayaishi O., Nozaki M. Nature and mechanisms of oxygenases. Science. 1969 Apr 25;164(3878):389–396. doi: 10.1126/science.164.3878.389. [DOI] [PubMed] [Google Scholar]
- Hirschberg R., Ensign J. C. Oxidation of nicotinic acid by a Bacillus species: purification and properties of nicotinic acid and 6-hydroxynicotinic acid hydroxylases. J Bacteriol. 1971 Nov;108(2):751–756. doi: 10.1128/jb.108.2.751-756.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein L. I., Dalton B. P. The hydroxylation of nicotine: the origin of the hydroxyl oxygen. Biochem Biophys Res Commun. 1965 Dec 21;21(6):644–648. doi: 10.1016/0006-291x(65)90535-8. [DOI] [PubMed] [Google Scholar]
- Holcenberg J. S., Tsai L. Nicotinic acid metabolism. IV. Ferredoxin-dependent reduction of 6-hydroxynicotinic acid to 6-oxo-1,4,5,6-tetrahydronicotinic acid. J Biol Chem. 1969 Mar 10;244(5):1204–1211. [PubMed] [Google Scholar]



