Abstract
We show that the heme-copper terminal oxidases of Thermus thermophilus (called ba3 and caa3) are able to catalyze the reduction of nitric oxide (NO) to nitrous oxide (N2O) under reducing anaerobic conditions. The rate of NO consumption and N2O production were found to be linearly dependent on enzyme concentration, and activity was abolished by enzyme denaturation. Thus, contrary to the eukaryotic enzyme, both T. thermophilus oxidases display a NO reductase activity (3.0 ± 0.7 mol NO/mol ba3 × min and 32 ± 8 mol NO/mol caa3 × min at [NO] ≈ 50 μM and 20°C) that, though considerably lower than that of bona fide NO reductases (300–4,500 mol NO/mol enzyme × min), is definitely significant. We also show that for ba3 oxidase, NO reduction is associated to oxidation of cytochrome b at a rate compatible with turnover, suggesting a mechanism consistent with the stoichiometry of the overall reaction. We propose that the NO reductase activity of T. thermophilus oxidases may depend on a peculiar CuB+ coordination, which may be revealed by the forthcoming three-dimensional structure. These findings support the hypothesis of a common phylogeny of aerobic respiration and bacterial denitrification, which was proposed on the basis of structural similarities between the Pseudomonas stutzeri NO reductase and the cbb3 terminal oxidases. Our findings represent functional evidence in support of this hypothesis.
Heme-copper terminal oxidases and bacterial NO reductases (NOR) were suggested to have originated during evolution from a common ancestor (1–3). The common phylogeny was proposed because of structural similarities between these enzymes (see ref. 4 for a review), notably in the large catalytic subunit, which displays significant sequence homology and conservation of crucial residues (including the six metal-binding histidines). The topology of the catalytic subunit of NOR (NorB) is predicted to comprise 12 transmembrane helices, as shown for subunit I of heme-copper oxidases (5, 6). Finally, the active site is, in both cases, a bimetallic center, consisting of a heme-iron and a second metal, which is Cu in oxidases and Fe in NOR (7, 8).
On the basis of these structural similarities, it was presumed that the mechanisms of O2 and NO reduction may share common features and, possibly, that O2 and NO may be used as alternative substrates by both enzyme families. The mechanism of NO reduction by NOR is, at present, largely hypothetical, which makes any comparison with the mechanism of O2 reduction by oxidases difficult. It is interesting, however, that a bacterial NOR with O2 reductase activity was found in Paracoccus denitrificans ATCC 35512 (9); in contrast, there is no unequivocal experimental evidence in support of the hypothesis that heme-copper oxidases catalyze the reduction of NO to N2O (2NO + 2e− + 2H+ → N2O + H2O). Brudwig et al. (10) reported that beef heart cytochrome c oxidase enhances (by a factor of 2) the reduction of NO by ascorbate and N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), but on a time scale of hours. The claim by Zhao et al. (11) that at low levels of NO and O2 the bovine enzyme catalyzes N2O production was contradicted by Stubauer et al. (12), who concluded that reduced beef heart cytochrome c oxidase in the presence of excess reductant displays no detectable NO reductase activity.
In this paper we show that, in contrast to the bovine enzyme, the two terminal oxidases purified from Thermus thermophilus (called ba3 and caa3; refs. 13 and 14) unequivocally display NO reductase activity in the presence of excess reductant, the reaction rate increasing with enzyme concentration and activity being lost upon denaturation. The evidence reported below is based on the rate of NO consumption, followed by spectrophotometric and amperometric methods, and on the rate of N2O production, detected by gas chromatography. In addition, we show by stopped-flow spectrophotometry that NO reduction is associated with the oxidation of cytochrome b in the ba3 enzyme at a rate consistent with turnover. Because CuB+ in both T. thermophilus oxidases displays unusually high affinity for gaseous ligands, notably CO (15, 16), we speculate that this property of the non-heme metal in the binuclear center is crucial for NO reduction. Our results are consistent with the hypothesis that heme-copper oxidases and NO reductases may have evolved from a common ancestor and support a possible coevolution of aerobic respiration and denitrification.
Materials and Methods
Materials.
Dodecyl-β-d-maltoside was purchased from Biomol (Plymouth Meeting, PA). Horse myoglobin (Mb), ascorbate, ruthenium(III) hexamine, TMPD, glucose oxidase, catalase, and phenazine methosulfate (PMS) were from Sigma. Experiments were performed in 0.1 M Hepes, pH 7.3/0.1% dodecyl-β-d-maltoside, unless otherwise stated. NO stock solutions were prepared by equilibrating in a tonometer degassed water with pure NO (Air Liquide, Paris) at 1 atm (1 atm = 101.3 kPa). The concentration of NO in NO-saturated water was measured by spectrophotometric titration of deoxy-human hemoglobin (Hb) and found to be 2.0 ± 0.1 mM.
Protein Purification.
Fermentation of Thermus thermophilus HB8 (ATCC 27634) has been performed at the Gesellschaft für Biotechnologische Forschung (Braunschweig, Germany). The purification of ba3 oxidase was described in ref. 16, and the preparation of the caa3 oxidase was described in ref. 17. Further details and a protein chemical description of the Thermus oxidases will be given elsewhere together with the crystallization of the two enzymes. Cytochrome c552 was purified from T. thermophilus as reported in ref. 18, bovine cytochrome c oxidase was purified according to ref. 19, and Hb was purified as reported in ref. 20. The concentration of these proteins was determined by using the following extinction coefficients: ɛ613 = 6.3 mM−1⋅cm−1 for reduced-minus-oxidized ba3 (14); ɛ605 = 11.7 mM−1⋅cm−1 for reduced-minus-oxidized caa3 (13); ɛ555 = 12.5 mM−1⋅cm−1 for deoxy-Hb and ɛ560 = 13.8 mM−1⋅cm−1 for deoxy-Mb (21); and ɛ605 = 22 mM−1⋅cm−1 for reduced-minus-oxidized bovine aa3 (22). The concentration of terminal oxidases is expressed in terms of functional unit, whereas the concentration of Hb and Mb refers to the heme content.
Time-Resolved Spectrophotometry.
These experiments were carried out by using a Durrum–Gibson apparatus equipped with a photodiode array (TN6500; Tracor Northern, Madison, WI). The instrument allows the collection of 1,024-element spectra as a function of time, with an acquisition time of 10 ms per spectrum and a 2-cm light path. Data analysis was performed by using matlab (Mathworks, Natick, MA), and noise filtering and spectral deconvolution were performed by using the singular value decomposition algorithm combined with curve-fitting procedures, according to ref. 23. Experimental spectra were fitted to linear combinations of reference spectra by using the “left division” option.
NO reductase activity was tested as follows. NO was added to an anaerobic solution of reduced oxidase (ba3, caa3, or beef heart aa3) in the presence of excess reductant, and NO concentration was monitored by mixing at different time intervals with deoxy-Hb or deoxy-Mb in excess over NO. To scavenge residual O2, 1 mM glucose was added to degassed buffers together with catalytic amounts of glucose oxidase and catalase. The concentration of NO at different times was measured from the concentration of Hb-NO at 100 ms after mixing (see ref. 12 for details). All experiments were carried out at 20°C unless otherwise stated.
NO Electrode.
Amperometric NO measurements were performed by using a Clark-type NO electrode (ISO-NO; World Precision Instruments, Sarasota, FL) interfaced with a chart recorder. The NO probe was connected to a 2.0-ml gas-tight chamber containing a degassed solution of reductants (2 mM ascorbate and 0.1 mM TMPD). At each measurement, aliquots of NO were added, reaching a final concentration of 0.3–17 μM NO. The observed initial drift in the NO concentration is caused by the chemical reaction of the reductants with NO. Then, oxidase (ba3, caa3, or aa3), previously reduced by ascorbate and TMPD, was injected and the NO disappearance was monitored. Measurements were performed under constant rapid stirring at 20°C. Catalytic activity was calculated by fitting the best tangent at [NO] = 10 μM and subtracting the initial NO decay (before enzyme addition).
Gas Chromatography.
Identification of N2O was achieved by gas chromatography using a dual column and valve system fitted to a Shimadzu GC-14B instrument. NO and N2O were separated on activated alumina and molecular sieve 13X columns and detected by a thermal conductivity cell (24). N2O (99.999%, grade UHP) for calibration was purchased from Messer-Griesheim (Krefeld, Germany). Head-space analysis was performed in 13-ml vials with 3 ml of reaction mixture and the gas phase comprising 4% NO in He. Electron donation was from ascorbate-reduced PMS. The standard reaction mixture consisted of 98 mM acetate buffer (pH 4.8), 32 mM sodium ascorbate, 0.17 mM PMS, 0.05% n-dodecyl β-d-maltoside, and up to 400 μl of an enzyme sample. The reaction mixture was incubated at 30°C, and at regular time intervals 50 μl of head space was analyzed for NO and N2O. Nonenzymatic N2O formation was checked at pH 4.8, 5.5, and 6.5, omitting the oxidase. An alternative assay system at neutral pH suppresses nonenzymatic N2O formation: 133 mM Tris⋅HCl, pH 7.1; 33 mM sodium ascorbate; 0.17 mM PMS; 0.05% dodecyl maltoside; 1 mM EDTA (25).
Results
NO Consumption by ba3 and caa3.
Contrary to the beef heart enzyme, which was shown (12) to be unable to consume NO in the presence of excess reductant, both terminal oxidases from T. thermophilus (ba3 and caa3) displayed NO reductase activity. As illustrated in Fig. 1 Upper, addition of ba3 is associated with the disappearance of NO, with the time course depicted in Fig. 1 Lower; the rate of NO consumption by ascorbate and ruthenium hexamine without added enzyme is much slower. We have shown that NO disappearance does not depend on the type of reductant, because the rate of the ba3-catalyzed NO consumption was the same, using either ascorbate plus ruthenium hexamine or ascorbate plus TMPD and cytochrome c552 as electron donors. More significant is the observation that the ba3-catalyzed NO consumption was abolished after denaturation of the enzyme by 30-min boiling in 1% SDS, which also leads to loss (>90%) of cytochrome c552 oxidase activity.
Figure 1.
NO reductase activity of ba3 and caa3. (Upper) ba3 (1.5 μM) was degassed, reduced by 10 mM ascorbate, 100 μM TMPD, 1 μM cytochrome c552, and, after addition of 60 μM NO, mixed in the stopped flow with 104 μM deoxy-Hb at the following incubation times: 0.9, 1.8, 3.4, 7.5, 10.8, 16, and 26.8 min. Difference spectra, collected at 100 ms after mixing, show NO disappearing from solution. Baseline, deoxy-Hb in the presence of ba3 and reductants before addition of NO; dashed spectrum, 104 μM NO-saturated Hb. (Lower) NO consumption as a function of time after addition of NO (60 μM final concentration) to: 10 mM ascorbate + 200 μM ruthenium hexamine (●) or 10 mM ascorbate + 10 μM TMPD (*); 5 μM (aa3) beef heart oxidase reduced by 10 mM ascorbate and 200 μM ruthenium hexamine (○); 1.5 μM ba3 reduced by 10 mM ascorbate + 200 μM ruthenium hexamine (□) or by 10 mM ascorbate + 10 μM TMPD + 1 μM cytochrome c552 (■); 0.3 μM caa3 reduced by 10 mM ascorbate and 10 μM TMPD (▴); or 100 μM TMPD (▵). Contrary to beef heart oxidase, both oxidases from T. thermophilus catalyze NO consumption significantly faster than reductants alone.
The caa3 oxidase of T. thermophilus catalyzed NO consumption more efficiently than ba3 (Fig. 1); because in these experiments TMPD was used as electron donor, the loss of NO in the presence of ascorbate plus TMPD was measured and found similar to the other controls. The NO reductase activity of caa3 was independent of TMPD concentration from 10 to 100 μM, a clear indication that the NO consumption rate is not limited by the reduction of caa3.
NO consumption by ba3 and caa3, measured at different enzyme concentrations (from 0.5 to 4.5 μM ba3 and from 0.1 to 1 μM caa3), was found to increase linearly as expected. From the initial rate we estimated the following activities: 3.0 ± 0.7 mol NO/mol ba3 × min at [NO] = 45 μM, and 32 ± 8 mol NO/mol caa3 × min at [NO] = 55 μM.
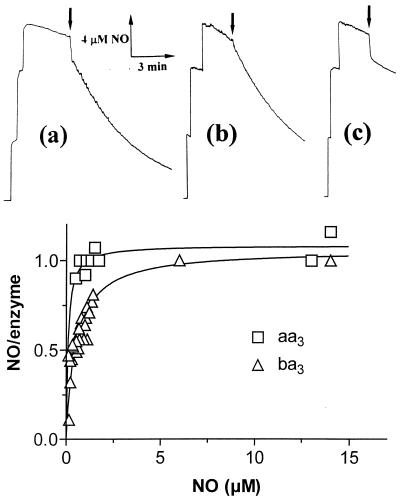
The NO reductase activity of ba3 and caa3 also was demonstrated by amperometry, using a NO electrode. In these experiments, an aliquot of the reduced enzyme (ba3, caa3, or bovine oxidase) was added anaerobically to an O2-free solution of NO, containing ascorbate and TMPD, and the concentration of NO was monitored amperometrically (Fig. 2). When the enzyme concentration was a significant fraction of the total NO (see Fig. 2 a and c), a fast drop of NO concentration was observed, because of binding to the reduced enzyme; from this amplitude the stoichiometry of NO bound to the reduced binuclear center can be assessed (Fig. 2 Lower). NO consumption after initial binding (Fig. 2 Upper) is consistent with a significant catalytic activity of ba3 and caa3 (Fig. 2 a and b), whereas the bovine enzyme binds NO, but does not catalyze its reduction (Fig. 2c; see also ref. 12). In agreement with the results depicted in Fig. 1, caa3 was more active than ba3; at [NO] = 10 μM, the estimated activity was 0.50 ± 0.01 mol NO/mol ba3 × min and 3.1 ± 0.3 mol NO/mol caa3 × min. Taking into account that NO concentration is very low in these experiments, these estimates are not inconsistent with those obtained optically (Fig. 1).
Figure 2.
Amperometric measurements: NO binding and catalytic consumption. (Upper) Three aliquots of NO (final concentration 15 μM) were added to degassed buffer containing 2 mM ascorbate and 0.1 mM TMPD before addition of reduced oxidases [see arrows; final concentrations: 2 μM ba3 (a); 0.2 μM caa3 (b); 2 μM aa3 (c)]. NO decay before addition of each enzyme is attributed to the reaction with the reductants. Upon addition of ba3 and caa3, a catalytic NO consumption (after initial binding seen as a vertical drop) was observed, whereas with beef heart oxidase only stoichiometric binding was seen. (Lower) Stoichiometry of NO binding to reduced ba3 (▵) and beef heart oxidase (□) as a function of NO concentration. For both enzymes, NO binds maximally with a 1:1 stoichiometry in the NO concentration range explored.
The stoichiometry of NO binding to reduced T. thermophilus ba3 and bovine aa3 was measured at different NO concentrations (from 0.3 to 15 μM). In spite of some difference in affinity, both oxidases in the reduced state bind 1 NO/enzyme over the whole NO concentration range. This result implies that, if the NO reductase activity of ba3 were to reside in its capability to bind a second NO at the binuclear center, the affinity of the putative second ligand would be low.
The Product of NO Reduction Is N2O.
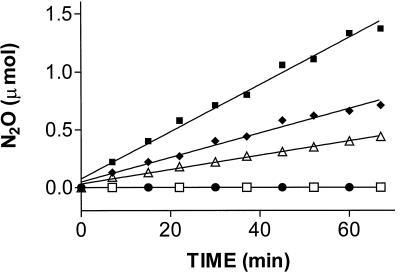
The product of NO reduction by the ba3 oxidase was identified by gas chromatography using synthetic N2O as the standard. The time course of N2O production followed by discontinuous sampling of the reaction mixture head space is shown in Fig. 3 at three different enzyme concentrations and at pH 4.8. The calculated enzymatic activity (3.0 ± 0.3 mol NO reduced/mol ba3 × min) was in agreement with the spectroscopic assay illustrated above. No N2O formation was observed upon omitting the enzyme or upon addition of ba3 boiled previously in 1% SDS (Fig. 3); this excludes that the enhanced rate of N2O production is caused by contamination. The assay also was carried out at pH 5.5 and 6.5; at the latter pH, nonenzymatic N2O formation contributed approximately 15% to the overall reduction rate. As a further control, we used a Tris-buffered system run at neutral pH but suppressing nonenzymatic NO reduction by the addition of 1 mM EDTA. As in the standard assay system, we found clear evidence that production of N2O depended on ba3 concentration and was not observed after denaturation of the enzyme. In summary, the chromatographic assay provided clear evidence for innate NO-reducing activity of the ba3 oxidase, yielding N2O as the product.
Figure 3.
N2O formation by the ba3 oxidase. The rate of N2O formation, identified by head-space analysis and gas chromatography, is shown at different enzyme concentrations in acetate buffer (pH 4.8) and ascorbate-reduced PMS. The low pH is necessary to suppress nonenzymatic N2O formation, which increases with pH and may become significant unless specific countermeasures are being taken (25). The graph combines data of duplicate experiments, which were fitted by linear regression at 4.94 (■), 2.47 (⧫), and 1.24 (▵) μM ba3, respectively; ●, enzyme boiled in 1% SDS for 5 min; □, assay mixture without the enzyme.
Oxidation of Reduced ba3 by NO.
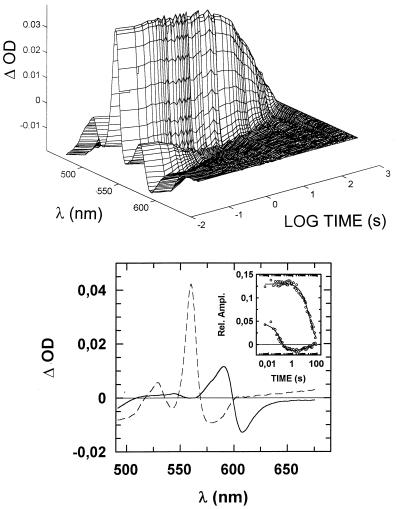
If NO can be used as an alternative electron acceptor by the terminal oxidases of T. thermophilus, these enzymes are expected to be oxidized by NO. We followed spectrophotometrically the oxidation of reduced ba3 by NO, performing the following experiment. An anaerobic solution of ba3 was reduced with ascorbate (1 mM) in the presence of 1 mM CO to drive reduction to completion. The resulting CO-bound and fully reduced ba3 was mixed anaerobically in the stopped-flow instrument with 250 μM NO, and visible spectra were collected as a function of time. The raw data (Fig. 4 Upper) clearly indicated that two time-resolved processes occur over the time interval explored (10 ms–80 s); a faster process, which could be detected easily at 580–620 nm, was followed by a slower one, clearly indicated by the loss of absorbance around 560 nm, the peak of reduced cytochrome b. When these data were processed by singular value decomposition and fitted to two exponential decays, the optical components shown in Fig. 4 (Lower) were deconvoluted. The faster component (k1 = 9 s−1) corresponds to CO being replaced by NO binding to reduced cytochrome a32+ (solid spectrum in Fig. 4 Lower). Replacement of CO by NO is expected on the basis of the higher affinity of cytochrome a32+ for NO. In the dark, NO binding should be rate-limited by the intrinsic dissociation rate constant of CO from cytochrome a32+, which was found to be k = 0.8 s−1 (16); however, the intense light beam of the diode-array stopped-flow causes photodissociation of CO at k1 = 10–13 s−1, as observed independently by performing the above experiment in the absence of NO (not shown), which explains the relatively fast CO replacement by NO. The interesting observation here is that although NO is replacing CO at the level of cytochrome a32+, cytochrome b does not change its redox state. Only on a time scale of many seconds is cytochrome b oxidized (dashed spectrum in Fig. 4 Lower) at a rate (k2 = 0.04 s−1 at [NO] = 125 μM) consistent with the turnover experiments illustrated above. We therefore may conclude that NO reduction to N2O by ba3 is rate-limited by the same event that controls the intramolecular electron transfer from reduced cytochrome b to NO, presumably involving the cytochrome a3-CuB site. An additional, intriguing observation is that cytochrome b was not completely oxidized even at 125 μM NO. To assess whether the extent of cytochrome b oxidized depends on NO concentration, the experiment just described was repeated at [NO] from 2.5 μM to 1 mM (after mixing). The results indicated that, although the rate of CO displacement from cytochrome a32+ (k1 ≈ 10 s−1) is independent of NO concentration, the amplitude of the slower cytochrome b oxidation increases at higher NO, yielding an apparent Kd ≈ 40 μM (data not shown).
Figure 4.
Time course of the oxidation of reduced ba3 by NO. ba3 (5 μM) was reduced anaerobically by 1 mM ascorbate in the presence of 1 mM CO, and the resulting CO-bound fully reduced enzyme was mixed with 250 μM NO at 20°C. (Upper) Absorption changes collected from 10 ms to 80 s after mixing. (Lower) Optical components deconvoluted by fitting the first two V columns of the singular value decomposition output (scaled by their relative singular values) to two exponential decays (Inset: best fit). The faster phase (solid spectrum) corresponds to CO displacement by NO, proceeding at k1 = 9 s−1, whereas cytochrome b oxidation (dashed spectrum) occurs at a much slower rate, k2 = 0.04 s−1, which is consistent with the turnover number measured for NO consumption by ba3.
The results described in this section are summarized as follows. Upon mixing CO-bound reduced ba3 with NO, (i) NO binds to reduced cytochrome a3 at the rate constant (k1 ≈ 10 s−1) of the light-induced CO dissociation from this site; (ii) cytochrome b is oxidized at a rate constant (k′ = 0.04 s−1) consistent with the NO consumption catalyzed by ba3; (iii) cytochrome a3 is invariably in the reduced NO-bound state (given the high NO concentration), and (iv) the extent of cytochrome b oxidation increases at higher NO, yielding an apparent Kd ≈ 40 μM.
Discussion
Aerobic respiration and denitrification were proposed to have a common phylogeny (1–3), largely based on structural similarities between the heme-copper oxidases and bacterial NOR. The former are the terminal enzymes of the aerobic electron transport chain and catalyze the reduction of O2 to H2O. NOR instead is responsible for the reduction of NO to N2O (2NO + 2e− + 2H+ → N2O + H2O), an intermediate step in the anaerobic electron transport chain of denitrifying bacteria, where nitrate is transformed into N2 (4, 26, 27).
Some relationships between O2 and NO reductases became apparent when the sequence of NorB from Pseudomonas stutzeri (28) was compared with the primary structure of the cbb3 oxidases, revealing unexpected similarities (1, 2). The cbb3 oxidases, which display the highest sequence homology with NOR, are preferentially expressed under microaerobic conditions and display an unusually high affinity for O2. Based on this and other evidence, it was proposed that denitrification and aerobic respiration may share a common phylogeny (see refs. 3 and 4 for reviews). In spite of some skepticism, it may be recalled that in the Earth's early times, when NO in the atmosphere was possibly more abundant than O2 (29), NOR might have emerged first, and only later, when the level of atmospheric O2 began to rise, “microaerobic” cbb3-like oxidases may have evolved from NOR, preceding the appearance of the present-day heme-copper oxidases selected when high O2 levels were achieved, because of oxygenic photosynthesis.
The hypothesis of an evolutionary link between O2 and NO reductases was based, until now, on structural similarities. Functional evidence was lacking, partly because the mechanism of NOR catalysis is largely hypothetical, whereas that of oxidases has been investigated extensively (see ref. 30 for a review). However, if the phylogenic hypothesis is correct, one may expect that both types of reductases may use both O2 and NO as alternative electron acceptors, though with different efficiency. Consistent with this view, NOR purified from Paracoccus denitrificans ATCC 35512 was found (9) to reduce O2 to H2O (Vmax ≈ 600 mol O2/mol enzyme × min), although with low affinity (Kd = 0.9 mM).
The results presented in this paper may be relevant to the above discussion insofar as both terminal oxidases of T. thermophilus, called ba3 and caa3, were found to catalyze the reduction of NO to N2O at a significant rate. The activity measured with ba3 (3.0 ± 0.7 mol NO/mol ba3 × min at [NO] = 45 μM) and caa3 (32 ± 8 mol NO/mol caa3 × min at [NO] = 55 μM) is much lower than that of bona fide NOR (ranging from 300 to 4,500 mol NO/mol NOR × min; see ref. 4), but probably underestimated because of the thermophilic nature of these oxidases (13, 16). In contrast to this observation, other extensively studied heme-copper oxidases do not display a believable NO reductase activity. In fact, over and above some earlier evidence (10, 11) summarized in the Introduction, we demonstrated previously (12) that the mitochondrial beef heart cytochrome c oxidase is unable to catalyze NO consumption at a detectable rate over tens of minutes, an observation confirmed in the present paper.
The active site of heme-copper terminal oxidases is designed evolutionary to react with O2, and, therefore, it is not surprising that the T. thermophilus enzymes reduce NO with a low apparent affinity, as indicated by the exponential time course of NO consumption (Fig. 1). It is interesting to notice a parallel observation by Fujiwara and Fukumori (9), who reported that O2 was consumed by NOR from Paracoccus denitrificans ATCC 35512 with low affinity and non-zero-order kinetics. We propose that NO reductase activity is sustained by the reaction of two NO molecules with the fully reduced binuclear active site of ba3, one binding very rapidly and with high affinity to reduced cytochrome a32+, and the other with very much lower affinity to CuB+, which donates an electron to NO and is rereduced by cytochrome b. This would account for the observation that the rate of oxidation of cytochrome b (k = 0.04 s−1) shown in Fig. 4 is consistent with the NO-consumption turnover number (0.06 ± 0.01 mol NO/mol ba3 × s) and seems to be associated to the rate-limiting step in catalysis. Moreover we estimated from these experiments that reduced CuB+ has a very low affinity for NO (Kd ≈ 40 μM). Given the finding that in the presence of excess NO, reduced cytochrome a3 is invariably in the NO-bound state, it seems reasonable to assume that this cytochrome as well plays a role in the bielectronic reduction of 2 NO to N2O, as demanded by the overall reaction.
Therefore, we have shown that in addition to a NOR capable of reducing O2 (9), there are heme-copper oxidases capable of reducing NO, albeit with low efficiency. The structural features that distinguish a NOR from an O2 reductase, however, are unclear. The sufficiently high sequence similarity of the catalytic subunits of these enzymes allowed for the building of a three-dimensional structural model combining the primary sequence of NorB from P. stutzeri and the crystallographic data of P. denitrificans oxidase (31). This model indicates that in these two families of enzymes, the general features of the active site, consisting of a bimetallic center containing a heme iron and a second metal, which is Cu in oxidase and Fe in NOR, are conserved.
The presence of a non-heme metal (Cu or Fe) as part of the active site therefore is a prerequisite for the catalytic activity of both NO and O2 reductases, as demonstrated by site-directed mutagenesis (31, 32). Sequence alignment suggests that the crosslink between one of the histidine ligands of CuB and a nearby tyrosine residue, initially discovered in the bovine oxidase (33), is absent in NOR and cbb3 oxidases (34). Therefore, a significant difference between NO and O2 reductases seems to be at the level of the non-heme metal-binding site, which may play the crucial role in determining whether O2 or NO is used as the preferential substrate. Although at present we cannot exclude that other terminal oxidases will display significant NO reductase activity, we notice that in both T. thermophilus enzymes, CuB+ was shown to display an unusually high affinity for CO compared with other oxidases (Ka = 103–104 M−1 vs. Ka = 101–102 M−1; refs. 15 and 16). In a previous study (16), we suggested that this feature may have a physiological role: the higher affinity may allow reduced CuB to act as an O2 trap in these thermophilic oxidases, which have to cope with a reduced O2 availability in the physiological environment of the thermophilic microorganisms, because of the reduced gas solubility at high temperature. We suggest that the higher affinity of reduced CuB for CO, if applicable to NO, may be an element to explain the observed NO reductase activity of the T. thermophilus oxidases.
If the efficiency of the catalytic formation of N2O depends on the affinity for NO of the non-heme metal in the binuclear center, the non-heme Fe in NOR should bind NO much more tightly than CuB+ in the oxidases, which, in the bovine enzyme, was shown to bind NO only at very high concentrations (10). The evolutionary transition between NOR and oxidases, implying among other features the replacement of Fe with Cu, may have produced enzymes with intermediate properties. The T. thermophilus oxidases may represent examples in which the non-heme metal is already Cu, which, in the reduced state, however, displays a sufficiently high affinity for gaseous ligands to sustain NO reduction. If the proposed higher affinity of CuB for O2 provides an adaptive advantage for microaerophilic organisms (16), we predict that the cbb3 oxidases (1, 2) also may catalyze the reduction of NO with significant efficiency.
In conclusion, we have presented experimental evidence that two heme-copper oxidases from a thermophilic microorganism are able to catalyze the reduction of 2 NO to N2O at a significant rate, supporting the hypothesis of a common evolutionary origin of aerobic respiration and bacterial denitrification.
Acknowledgments
G.B. and T.S. thank M. Tatarek and H. Didden, and W.G.Z. thanks B. Schreckenberger for skillful technical assistance. The contributions of Dr. Elena Forte and of Emilio D'Itri are gratefully acknowledged. Fermentation of T. thermophilus at the Gesellschaft für Biotechnologische Forschung, Braunschweig, is acknowledged. This work is supported partially by Ministero dell'Università e della Ricerca Scientifica e Tecnologica of Italy (PRIN “Bioenergetica e Trasporto di Membrane” to P.S.), by the Deutsche Forschungsgemeinschaft (Bu 463/3 to G.B. and Zu 29/14-1 to W.G.Z.), and by Fonds der Chemischen Industrie (to W.G.Z.). G.S. was supported by a Fellowship from FWF Austria (Erwin-Schroedinger-Scholarship J01475).
Abbreviations
- NOR
bacterial NO reductase
- TMPD
N,N,N′,N′-tetramethyl-p-phenylenediamine
- PMS
phenazine methosulfate
- Hb
human hemoglobin
- Mb
horse myoglobin
References
- 1.Van der Oost J, de Boer A P N, de Gier J L, Zumft W G, Stouthamer A H, Spanning R J M. FEMS Microbiol Lett. 1994;121:1–10. doi: 10.1111/j.1574-6968.1994.tb07067.x. [DOI] [PubMed] [Google Scholar]
- 2.Saraste M, Castresana J. FEBS Lett. 1994;341:1–4. doi: 10.1016/0014-5793(94)80228-9. [DOI] [PubMed] [Google Scholar]
- 3.Hendriks J, Gohlke U, Saraste M. J Bioenerg Biomembr. 1998;30:15–24. doi: 10.1023/a:1020547225398. [DOI] [PubMed] [Google Scholar]
- 4.Zumft W G. Microbiol Mol Biol Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata S, Ostermeier C, Ludwig B, Michel H. Nature (London) 1995;376:660–669. doi: 10.1038/376660a0. [DOI] [PubMed] [Google Scholar]
- 6.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 7.Cheesman M R, Zumft W G, Thomson A J. Biochemistry. 1998;37:3994–4000. doi: 10.1021/bi972437y. [DOI] [PubMed] [Google Scholar]
- 8.Hendriks J, Warne A, Gohlke U, Haltia T, Ludovici C, Lübben M, Saraste M. Biochemistry. 1998;37:13102–13109. doi: 10.1021/bi980943x. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara T, Fukumori Y. J Bacteriol. 1996;178:1866–1871. doi: 10.1128/jb.178.7.1866-1871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brudwig G W, Stevens T H, Chan S I. Biochemistry. 1980;19:5275–5285. doi: 10.1021/bi00564a020. [DOI] [PubMed] [Google Scholar]
- 11.Zhao X, Sampath V, Caughey W S. Biochem Biophys Res Commun. 1995;212:1054–1060. doi: 10.1006/bbrc.1995.2076. [DOI] [PubMed] [Google Scholar]
- 12.Stubauer G, Giuffrè A, Brunori M, Sarti P. Biochem Biophys Res Commun. 1998;245:459–465. doi: 10.1006/bbrc.1998.8457. [DOI] [PubMed] [Google Scholar]
- 13.Fee J A, Choc M G, Findling K L, Lorence R, Yoshida T. Proc Natl Acad Sci USA. 1980;77:147–151. doi: 10.1073/pnas.77.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zimmermann B H, Nitsche C I, Fee J A, Rusnak F, Münck E. Proc Natl Acad Sci USA. 1988;85:5779–5783. doi: 10.1073/pnas.85.16.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodruff W H. J Bioenerg Biomembr. 1993;25:177–188. doi: 10.1007/BF00762859. [DOI] [PubMed] [Google Scholar]
- 16.Giuffrè A, Forte E, Antonini G, D'Itri E, Brunori M, Soulimane T, Buse G. Biochemistry. 1999;38:1057–1065. doi: 10.1021/bi9815389. [DOI] [PubMed] [Google Scholar]
- 17.Gerscher S, Hildebrandt P, Soulimane T, Buse G. Biospectroscopy. 1998;4:365–377. doi: 10.1002/(SICI)1520-6343(1998)4:6%3C365::AID-BSPY2%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 18.Soulimane T, von Walter M, Hof P, Than M E, Huber R, Buse G. Biochem Biophys Res Commun. 1997;237:572–576. doi: 10.1006/bbrc.1997.7041. [DOI] [PubMed] [Google Scholar]
- 19.Soulimane T, Buse G. Eur J Biochem. 1995;227:588–595. doi: 10.1111/j.1432-1033.1995.tb20429.x. [DOI] [PubMed] [Google Scholar]
- 20.Rossi Fanelli A, Antonini E, Caputo A. J Biol Chem. 1961;236:165–168. [PubMed] [Google Scholar]
- 21.Antonini E, Brunori M. In: Hemoglobin and Myoglobin in Their Reactions with Ligands. Neuberger A, Tatum E L, editors. Amsterdam: North–Holland; 1971. pp. 16–20. [Google Scholar]
- 22.Yonetani T. J Biol Chem. 1961;236:1680–1688. [PubMed] [Google Scholar]
- 23.Henry E, Hofrichter J. Methods Enzymol. 1992;210:129–192. [Google Scholar]
- 24.Frunzke K, Zumft W G. J Chromatogr. 1984;299:477–483. [Google Scholar]
- 25.Zumft W G, Frunzke K. Biochim Biophys Acta. 1982;681:459–468. doi: 10.1016/0005-2728(82)90188-8. [DOI] [PubMed] [Google Scholar]
- 26.Averill B A. Chem Rev. 1996;96:2951–2964. doi: 10.1021/cr950056p. [DOI] [PubMed] [Google Scholar]
- 27.Cutruzzolà F. Biochim Biophys Acta. 1999;1411:231–249. doi: 10.1016/s0005-2728(99)00017-1. [DOI] [PubMed] [Google Scholar]
- 28.Zumft W G, Braun C, Cuypers H. Eur J Biochem. 1994;219:481–490. doi: 10.1111/j.1432-1033.1994.tb19962.x. [DOI] [PubMed] [Google Scholar]
- 29.Kasting J F. Science. 1993;259:920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- 30.Babcock G T, Wikström M. Nature (London) 1992;356:301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 31.Kannt A, Michel H, Cheesmn M R, Thomson A J, Dreusch A B, Körner H, Zumft W G. In: Biological Electron Transfer Chains: Genetics, Composition and Mode of Operation. Canters G W, Vijgenboom E, editors. Dordrecht, The Netherlands: Kluwer; 1997. pp. 279–291. [Google Scholar]
- 32.Hosler J P, Ferguson-Miller S, Calhoun M W, Thomas J W, Hill J, Lemieux L, Ma J, Georgiou C, Fetter J, Shapleigh J, et al. J Bioenerg Biomembr. 1993;25:121–136. doi: 10.1007/BF00762854. [DOI] [PubMed] [Google Scholar]
- 33.Yoshikawa S, Shinzawa-Itoh K, Nakashima R, Yaono R, Yamashita E, Inoue N, Yao M, Fei M J, Libeu C P, Mizushima T, et al. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 34.Buse G, Soulimane T, Dewor M, Meyer H E, Blüggel M. Protein Sci. 1999;8:985–990. doi: 10.1110/ps.8.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]