Abstract
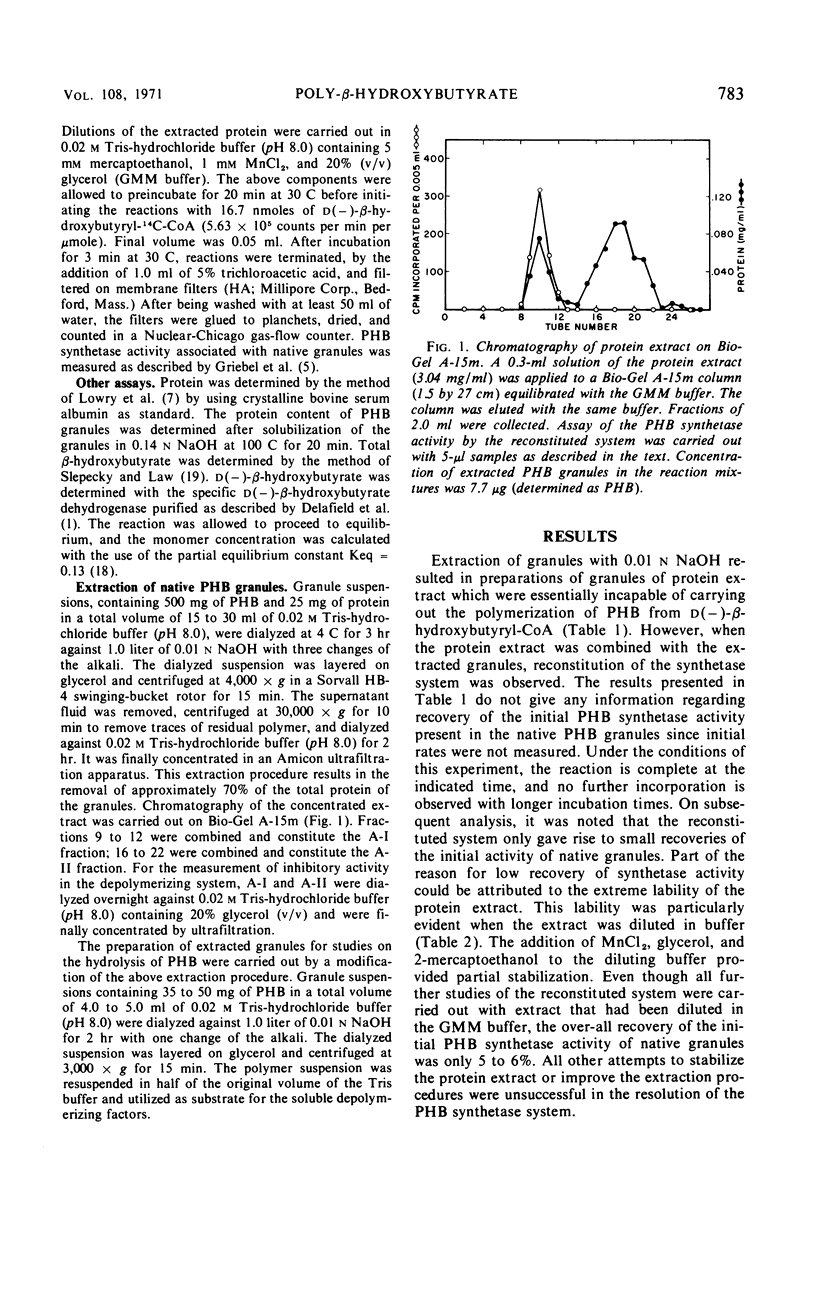
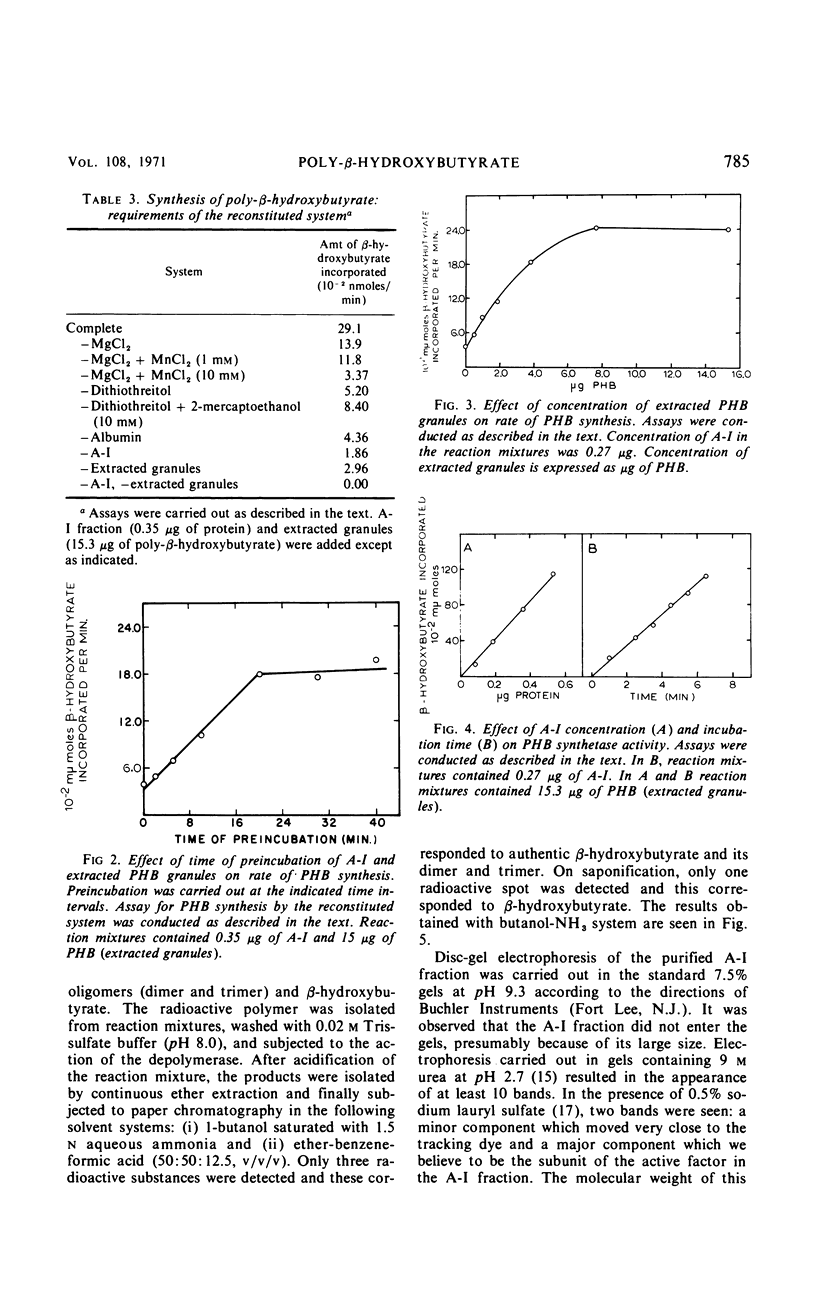
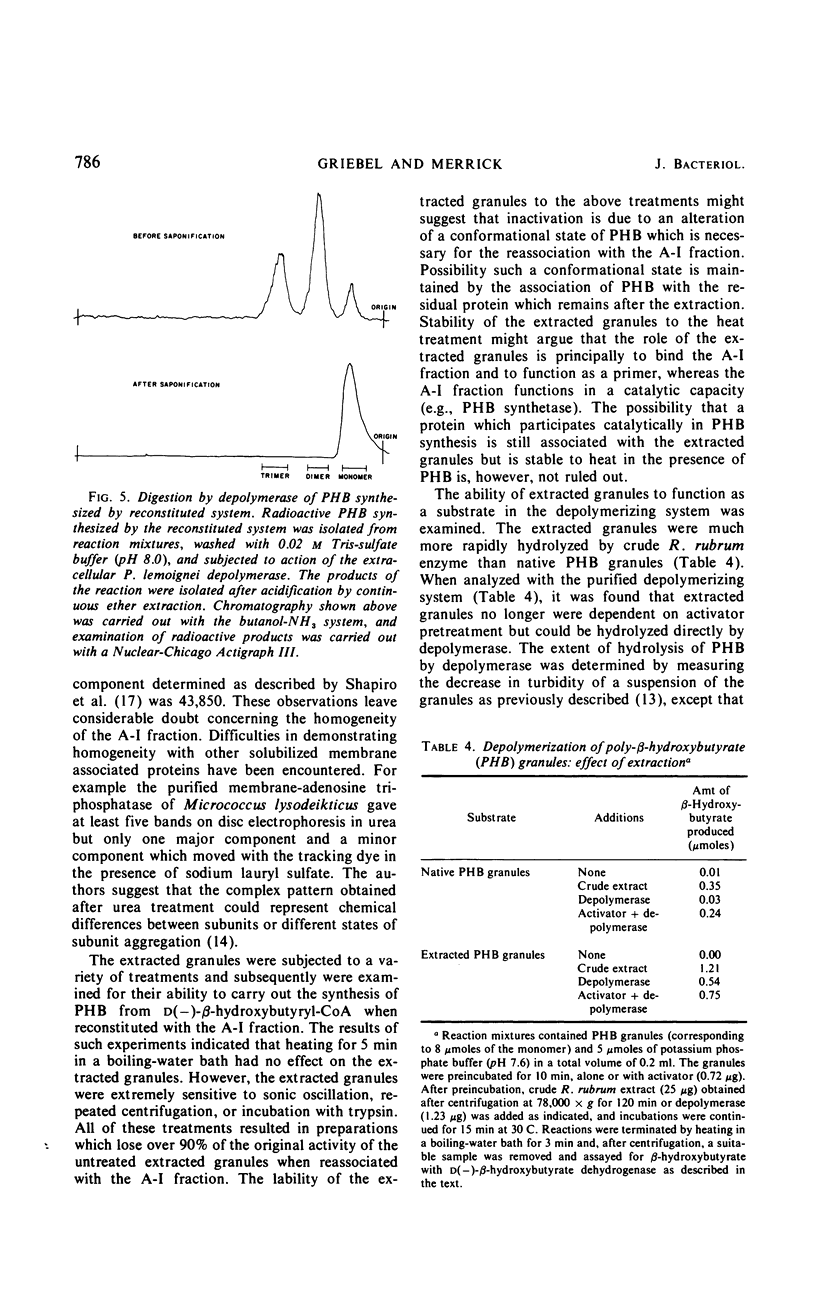
Mild alkaline extraction of native poly-β-hydroxybutyrate (PHB) granules results in the solubilization of a protein fraction. Both the solubilized protein fraction and the extracted granules are essentially devoid of PHB synthetase activity unless recombined. The protein fraction has been separated by chromatography into two components (A-I and A-II). A-I but not A-II can be recombined with extracted granules to give rise to PHB synthetase activity. Extracted granules no longer require pretreatment with activator or trypsin but are directly susceptible to hydrolysis by Rhodospirillum rubrum depolymerase. Addition of A-II or A-I prevents the direct hydrolysis by depolymerase. The inhibition is reversed by activator or trypsin. We conclude that native granules are associated with a protein inhibitor which prevents the hydrolysis of PHB by depolymerase unless the protein is destroyed by trypsin, removed by alkaline extraction, or modified by activator.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Delafield F. P., Cooksey K. E., Doudoroff M. beta-Hydroxybutyric dehydrogenase and dimer hydrolase of Pseudomonas lemoignei. J Biol Chem. 1965 Oct;240(10):4023–4028. [PubMed] [Google Scholar]
- Ellar D., Lundgren D. G., Okamura K., Marchessault R. H. Morphology of poly-beta-hydroxybutyrate granules. J Mol Biol. 1968 Aug 14;35(3):489–502. doi: 10.1016/s0022-2836(68)80009-9. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen D. W., Holtzer A. The substructure of the myosin molecule. Production and properties of the alkali subunits. Biochemistry. 1968 Nov;7(11):3935–3950. doi: 10.1021/bi00851a022. [DOI] [PubMed] [Google Scholar]
- Griebel R., Smith Z., Merrick J. M. Metabolism of poly-beta-hydroxybutyrate. I. Purification, composition, and properties of native poly-beta-hydroxybutyrate granules from Bacillus megaterium. Biochemistry. 1968 Oct;7(10):3676–3681. doi: 10.1021/bi00850a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUNDGREN D. G., PFISTER R. M., MERRICK J. M. STRUCTURE OF POLY-BETA-HYDROXYBUTYRIC ACID GRANULES. J Gen Microbiol. 1964 Mar;34:441–446. doi: 10.1099/00221287-34-3-441. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Doudoroff M. Poly-beta-hydroxybutyrate depolymerases of Pseudomonas lemoignei. Proc Natl Acad Sci U S A. 1966 Sep;56(3):960–965. doi: 10.1073/pnas.56.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. DEPOLYMERIZATION OF POLY-BETA-HYDROXYBUTYRATE BY INTRACELLULAR ENZYME SYSTEM. J Bacteriol. 1964 Jul;88:60–71. doi: 10.1128/jb.88.1.60-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRICK J. M., DOUDOROFF M. Enzymatic synthesis of poly-beta-hydroxybutyric acid in bacteria. Nature. 1961 Mar 18;189:890–892. doi: 10.1038/189890a0. [DOI] [PubMed] [Google Scholar]
- MERRICK J. M., LUNDGREN D. G., PFISTER R. M. MORPHOLOGICAL CHANGES IN POLY-BETA-HYDROXYBUTYRATE GRANULES ASSOCIATED WITH DECREASED SUSCEPTIBILITY TO ENZYMATIC HYDROLYSIS. J Bacteriol. 1965 Jan;89:234–239. doi: 10.1128/jb.89.1.234-239.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Tzagoloff A. Studies on the mitochondrial adenosine triphosphatase system. IV. Purification and characterization of the oligomycin sensitivity conferring protein. Biochemistry. 1968 Apr;7(4):1603–1610. doi: 10.1021/bi00844a050. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Neville D. M., Jr Fractionation of cell membrane protein by disc electrophoresis. Biochim Biophys Acta. 1967 Jan 18;133(1):168–170. doi: 10.1016/0005-2795(67)90051-7. [DOI] [PubMed] [Google Scholar]
- SHUSTER C. W., DOUDOROFF M. A cold-sensitive D(-) beta-hydroxybutyric acid dehydrogenase from Rhodospirillum rubrum. J Biol Chem. 1962 Feb;237:603–607. [PubMed] [Google Scholar]
- Samejima T., McCabe W. J., Yang J. T. Reconstitution of alkaline-denatured catalase. Arch Biochem Biophys. 1968 Sep 20;127(1):354–360. doi: 10.1016/0003-9861(68)90236-1. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]


