Abstract
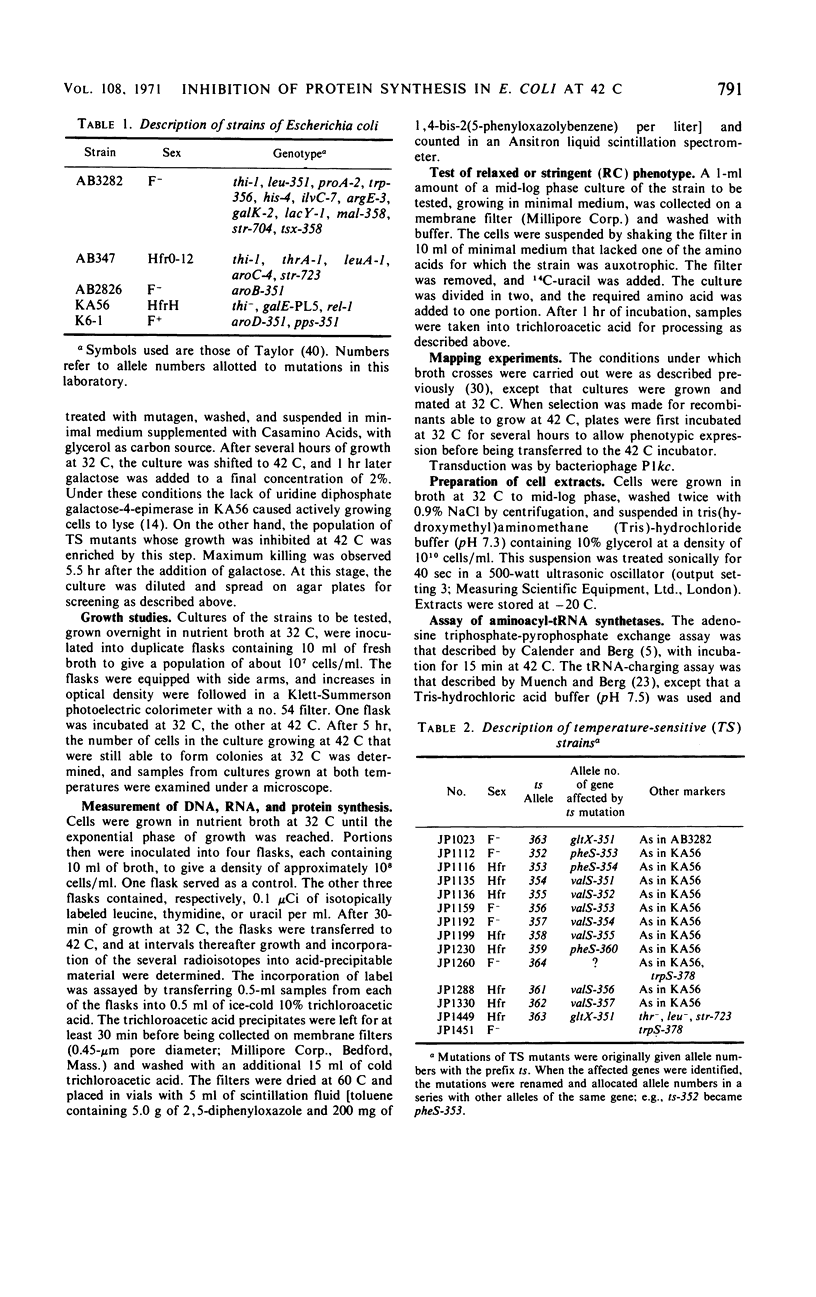
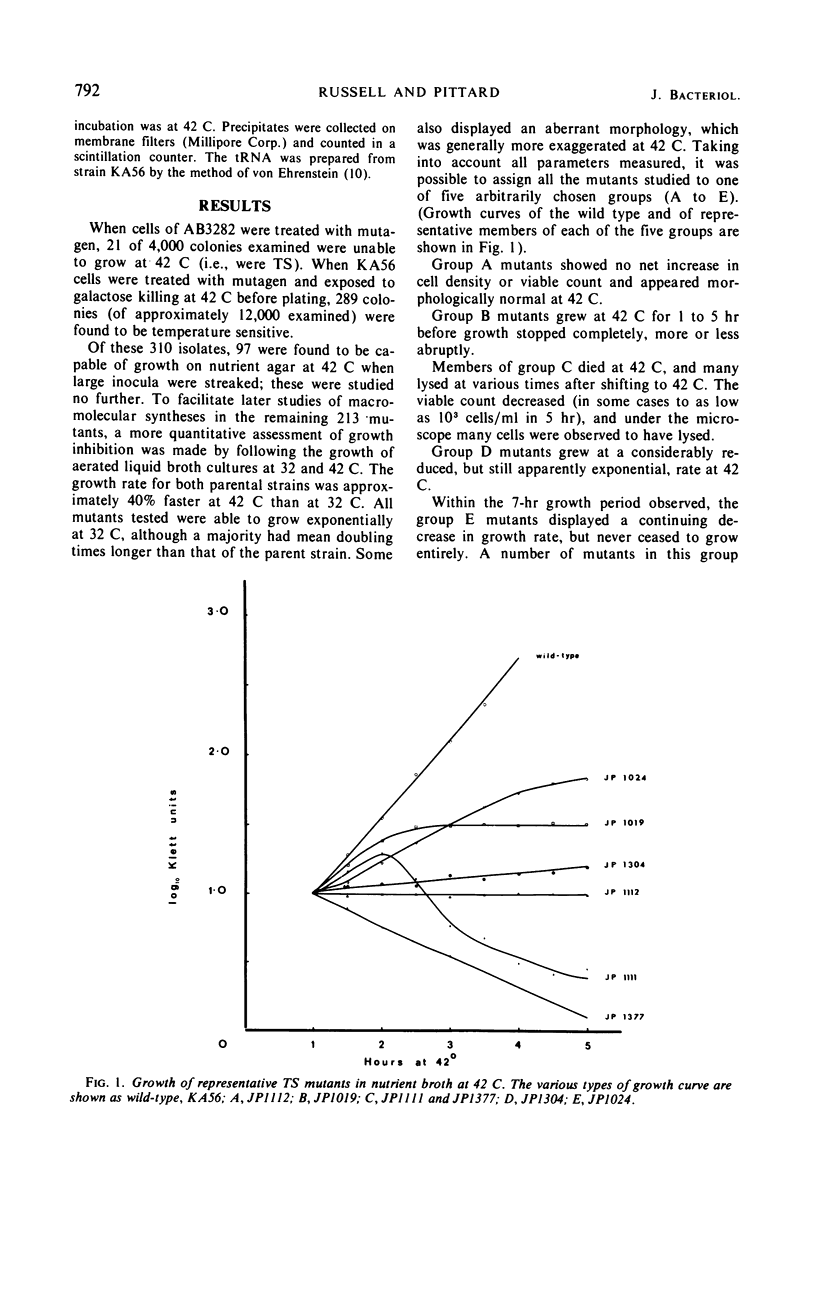
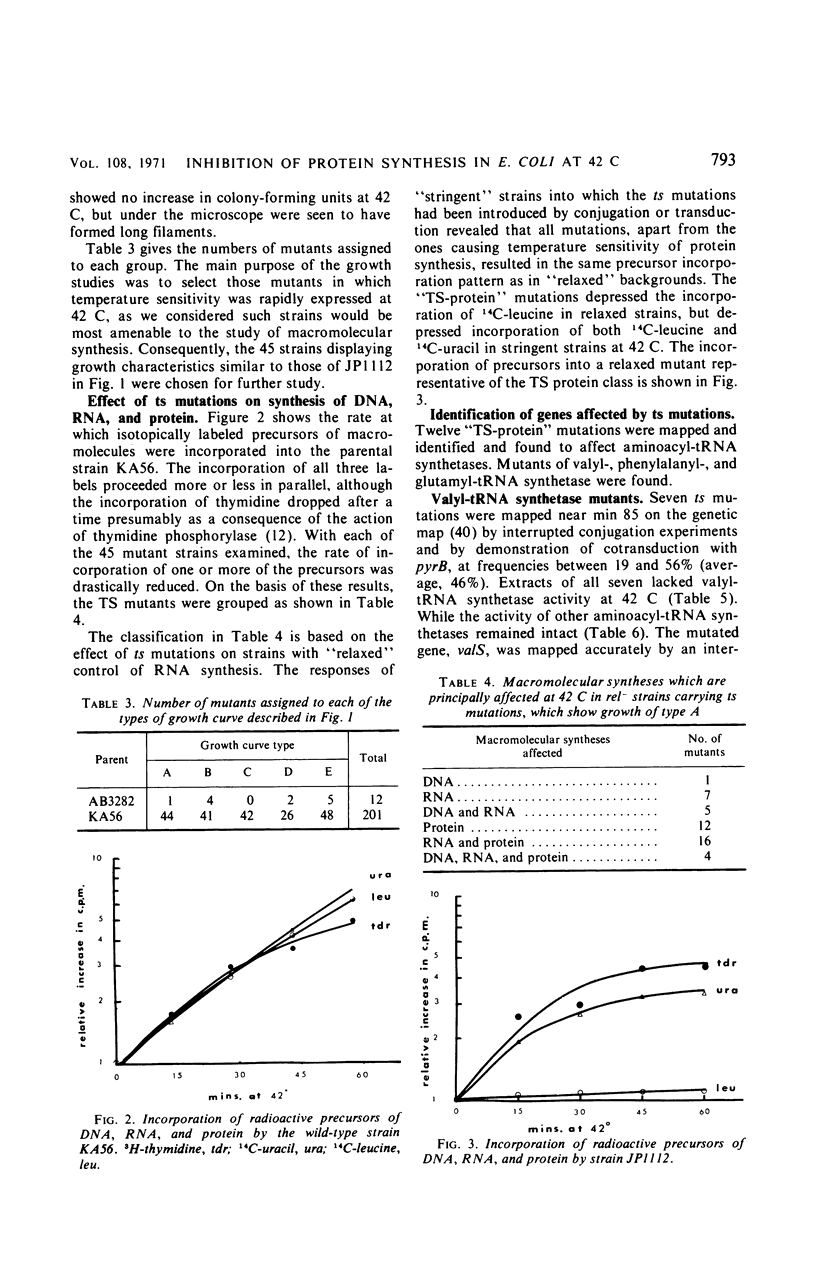
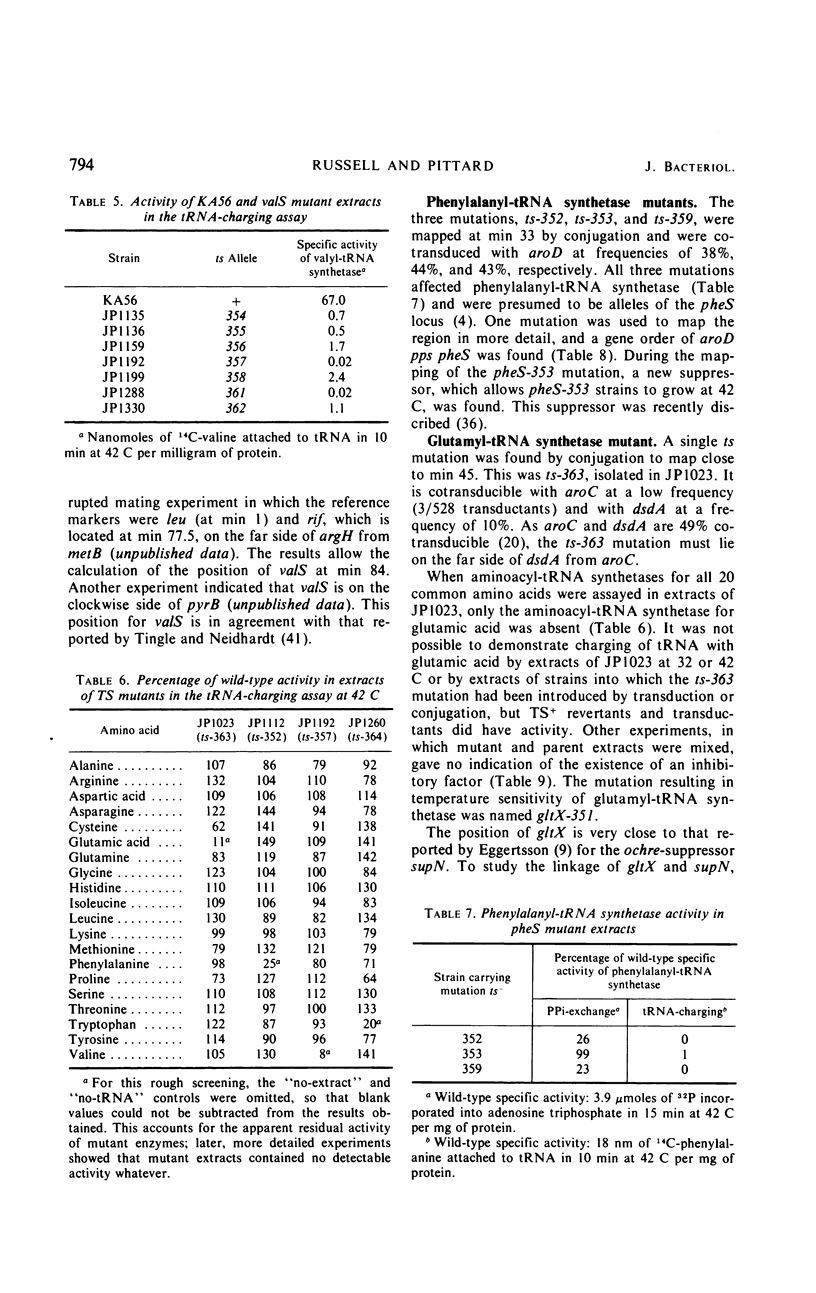
Members of a collection of mutants of Escherichia coli unable to form colonies on nutrient agar at 42 C have been characterized on the basis of their growth response to a shift from 32 to 42 C in liquid medium. Forty-four mutants, which show an abrupt, nonlethal cessation of growth when moved to the restrictive temperature, have been characterized with respect to the effect of the mutation responsible for temperature sensitivity on deoxyribonucleic acid, ribonucleic acid, and protein synthesis. In 12 mutants, the mutation causing temperature sensitivity of growth primarily affects protein synthesis, in each case through an altered aminoacyl-transfer ribonucleic acid synthetase. Mutants with temperature-sensitive glutamyl-, phenylalanyl-, and valyl-transfer ribonucleic acid synthetases have been obtained, and the genes specifying these enzymes have been mapped by conjugation and transduction. Another mutant has been shown to possess a temperature-sensitive tryptophanyl-transfer ribonucleic acid synthetase, but this is not responsible for inability to grow at 42 C on media containing tryptophan.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., BURNS S. N. Genetic variation in the sex factor of Escherichia coli. J Bacteriol. 1960 Mar;79:321–330. doi: 10.1128/jb.79.3.321-330.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böck A. Mutation affecting the charging reaction of alanyl-tRNA synthetase from Escherichia coli K 10. Arch Mikrobiol. 1969 Oct;68(2):165–178. doi: 10.1007/BF00413875. [DOI] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Genetic mapping of phenylalanyl-sRNA synthetase in Escherichia coli. Science. 1967 Jul 7;157(3784):78–79. doi: 10.1126/science.157.3784.78. [DOI] [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- Cassio D., Waller J. P. Etude la méthionyl-tRNA synthétase d'Escherichia coli. 3. Dissociation en sous-unités actives par action d'un facteur extrinsèque. Eur J Biochem. 1968 Jun;5(1):33–41. doi: 10.1111/j.1432-1033.1968.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Cooper P. H., Hirshfield I. N., Maas W. K. Map location of arginyl-tRNA synthetase mutations in Escherichia coli K-12. Mol Gen Genet. 1969 Aug 15;104(4):383–390. doi: 10.1007/BF00334238. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggertsson G. Mapping of ochre suppressors in Escherichia coli. Genet Res. 1968 Feb;11(1):15–20. doi: 10.1017/s0016672300011150. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. Nature. 1959 Oct 10;184(Suppl 15):1168–1169. doi: 10.1038/1841168a0. [DOI] [PubMed] [Google Scholar]
- Fangman W. L., Novick A. Mutant bacteria showing efficient utilization of thymidine. J Bacteriol. 1966 Jun;91(6):2390–2391. doi: 10.1128/jb.91.6.2390-2391.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk W. R., Berg P. Isolation and partial characterization of Escherichia coli mutants with altered glycyl transfer ribonucleic acid synthetases. J Bacteriol. 1970 Apr;102(1):193–203. doi: 10.1128/jb.102.1.193-203.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. P., Wilhelm R. C., Konigsberg W., Katze J. R. A structural gene for seryl-tRNA synthetase in Escherichia coli K12. J Mol Biol. 1970 Feb 14;47(3):619–625. doi: 10.1016/0022-2836(70)90332-3. [DOI] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Matsushiro A., Shimura Y. Isolation of the novel regulatory mutants of the tryptophan biosynthetic system in Escherichia coli. Mol Gen Genet. 1968;102(1):15–26. doi: 10.1007/BF00341866. [DOI] [PubMed] [Google Scholar]
- Kaplan S., Anderson D. Selection of temperature-sensitive activating enzyme mutants in Escherichia coli. J Bacteriol. 1968 Mar;95(3):991–997. doi: 10.1128/jb.95.3.991-997.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZZARINI R. A., MEHLER A. H. SEPARATION OF SPECIFIC GLUTAMATE- AND GLUTAMINE-ACTIVATING ENZYMES FROM ESCHERICHIA COLI. Biochemistry. 1964 Oct;3:1445–1449. doi: 10.1021/bi00898a009. [DOI] [PubMed] [Google Scholar]
- McFall E. Mapping of the d-serine deaminase region in Escherichia coli K-12. Genetics. 1967 Jan;55(1):91–99. doi: 10.1093/genetics/55.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R. Amber suppression and activating enzymes. J Mol Biol. 1966 Apr;16(2):556–561. doi: 10.1016/s0022-2836(66)80192-4. [DOI] [PubMed] [Google Scholar]
- Mitra S. K., Chakraburtty K., Mehler A. H. Binding of transfer RNA and arginine to the arginine transfer RNA synthetase of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):139–156. doi: 10.1016/0022-2836(70)90382-7. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Adelberg E. A. Mutants of Escherichia coli K-12 with an altered glutamyl-transfer ribonucleic acid synthetase. J Bacteriol. 1970 Jul;103(1):178–183. doi: 10.1128/jb.103.1.178-183.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgola E. J., Adelberg E. A. Streptomycin-suppressible lethal mutations in Escherichia coli. J Bacteriol. 1970 Jul;103(1):20–26. doi: 10.1128/jb.103.1.20-26.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Mol Gen Genet. 1967;100(2):216–224. doi: 10.1007/BF00333608. [DOI] [PubMed] [Google Scholar]
- Nass G., Stöffler G. Molecular weight distribution of the aminoacyl-tRNA-synthetases of Escherichia coli by gel filtration. Mol Gen Genet. 1967;100(4):378–382. doi: 10.1007/BF00334065. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrem D. L., Berg P. Glycyl-tRNA synthetase: an oligomeric protein containing dissimilar subunits. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1967–1974. doi: 10.1073/pnas.67.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preddie E. C. Tryptophanyl transfer ribonucleic acid synthetase from bovine pancreas. II. The chemically different subunits. J Biol Chem. 1969 Jul 25;244(14):3958–3968. [PubMed] [Google Scholar]
- Printz D. B., Gross S. R. An apparent relationship between mistranslation and an altered leucyl-tRNA synthetase in a conditional lethal mutant of Neurospora crassa. Genetics. 1967 Mar;55(3):451–467. doi: 10.1093/genetics/55.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVEL J. M., WANG S. F., HEINEMEYER C., SHIVE W. GLUTAMYL AND GLUTAMINYL RIBONUCLEIC ACID SYNTHETASES OF ESCHERICHIA COLI W. SEPARATION, PROPERTIES, AND STIMULATION OF ADENOSINE TRIPHOSPHATE-PYROPHOSPHATE EXCHANGE BY ACCEPTOR RIBONUCLEIC ACID. J Biol Chem. 1965 Jan;240:432–438. [PubMed] [Google Scholar]
- Rouget P., Chapeville F. Leucyl-tRNA synthetase. Purification of two interconvertible forms and evidence for an interconversion factor. Eur J Biochem. 1970 Jul;14(3):498–508. doi: 10.1111/j.1432-1033.1970.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Roy K. L., Söll D. Purification of five serine transfer ribonucleic acid species from Escherichia coli and their acylation by homologous and heterologous seryl transfer ribonucleic acid synthetases. J Biol Chem. 1970 Mar 25;245(6):1394–1400. [PubMed] [Google Scholar]
- Russell R. R., Pittard A. J. New suppresor in Escherichia coli. J Bacteriol. 1971 Sep;107(3):736–740. doi: 10.1128/jb.107.3.736-740.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Nester E. W. Mutants of Escherichia coli with an altered tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1969 Oct;100(1):167–175. doi: 10.1128/jb.100.1.167-175.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Szentirmai M., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XV. Biochemical properties of mutants resistant to thiaisoleucine. J Bacteriol. 1968 May;95(5):1672–1679. doi: 10.1128/jb.95.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söll D. Studies on polynucleotides. LXXXV. Partial purification of an amber supressor tRNA and studies on in vitro suppression. J Mol Biol. 1968 May 28;34(1):175–187. doi: 10.1016/0022-2836(68)90243-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Neidhardt F. C. Mapping of a structural gene for valyl-transfer ribonucleic acid synthetase in Escherichia coli by transduction. J Bacteriol. 1969 May;98(2):837–839. doi: 10.1128/jb.98.2.837-839.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocchini-Valentini G. P., Felicetti L., Rinaldi G. M. Mutants of Escherichia coli blocked in protein synthesis: mutants with an altered G factor. Cold Spring Harb Symp Quant Biol. 1969;34:463–468. doi: 10.1101/sqb.1969.034.01.052. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Jacob F., Gros F. Mutations thermosensibles des systèmes activant la valine chez E. coli. Bull Soc Chim Biol (Paris) 1965;47(8):1609–1626. [PubMed] [Google Scholar]



