Abstract
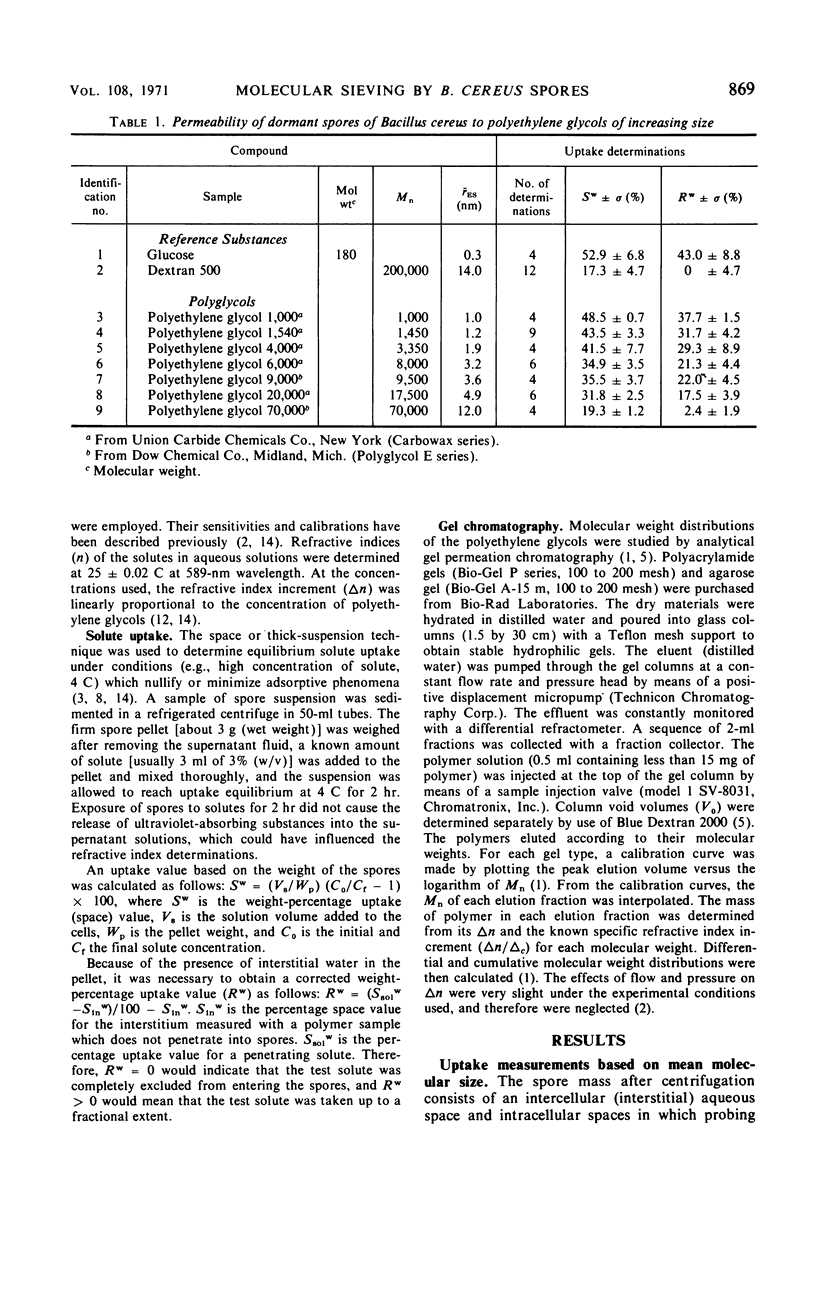
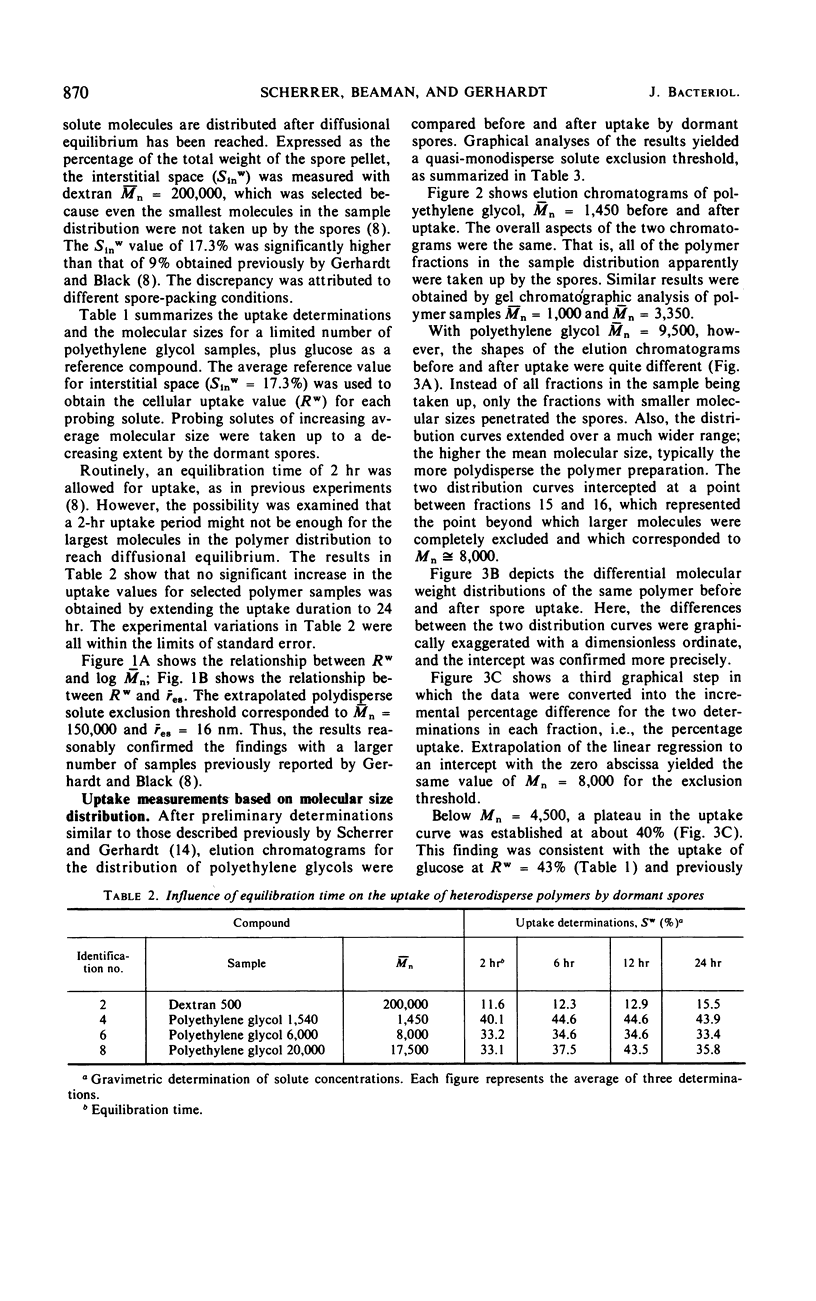
The threshold surface porosity in the dormant spore of Bacillus cereus strain T was assessed by measuring passive permeabilities to a series of polydisperse polyethylene glycol samples which increased in average molecular size. The apparent exclusion threshold at diffusional equilibrium corresponded to a polymer of number-average molecular weight (¯Mn) = 150,000 and equivalent hydrodynamic radius (¯rES) = 16 nm, which confirmed a previous report. However, analytical gel chromatography before and after uptake by the spores revealed that only the low molecular weight fractions in a polymer sample distribution were taken up. From graphical analyses of the changes in molecular weight distributions, a quasi-monodisperse exclusion threshold was determined corresponding to Mn = 8,000 and rES = 3.2 nm. Thus, the equivalent porosity in the limiting outer integument appeared much more restrictive than heretofore shown for spores, although still more open than the monodisperse equivalent for the cell wall of vegetative bacilli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. I. Characterization of glucose uptake. J Bacteriol. 1961 Nov;82:743–749. doi: 10.1128/jb.82.5.743-749.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., JUDGE J. A. POROSITY OF ISOLATED CELL WALLS OF SACCHAROMYCES CEREVISIAE AND BACILLUS MEGATERIUM. J Bacteriol. 1964 Apr;87:945–951. doi: 10.1128/jb.87.4.945-951.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOULD G. W., HITCHINS A. D. SENSITIZATION OF BACTERIAL SPORES TO LYSOZYME AND TO HYDROGEN PEROXIDE WITH AGENTS WHICH RUPTURE DISULPHIDE BONDS. J Gen Microbiol. 1963 Dec;33:413–423. doi: 10.1099/00221287-33-3-413. [DOI] [PubMed] [Google Scholar]
- Matz L. L., Beaman T. C., Gerhardt P. Chemical composition of exosporium from spores of Bacillus cereus. J Bacteriol. 1970 Jan;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERRER R., GERHARDT P. MOLECULAR SIEVING BY CELL MEMBRANES OF BACILLUS MEGATERIUM. Nature. 1964 Nov 14;204:649–650. doi: 10.1038/204649a0. [DOI] [PubMed] [Google Scholar]
- Scherrer R., Gerhardt P. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J Bacteriol. 1971 Sep;107(3):718–735. doi: 10.1128/jb.107.3.718-735.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. A new approach to molecular configuration applied to aqueous pore transport. J Gen Physiol. 1967 Dec;50(11):2565–2578. doi: 10.1085/jgp.50.11.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Baillie A., Thomson R. O., Batty I. The use of ferritin labelled antibodies in the location of spore and vegetative antigens of Bacillus cereus. J Appl Bacteriol. 1966 Dec;29(3):512–518. doi: 10.1111/j.1365-2672.1966.tb03502.x. [DOI] [PubMed] [Google Scholar]



