Abstract
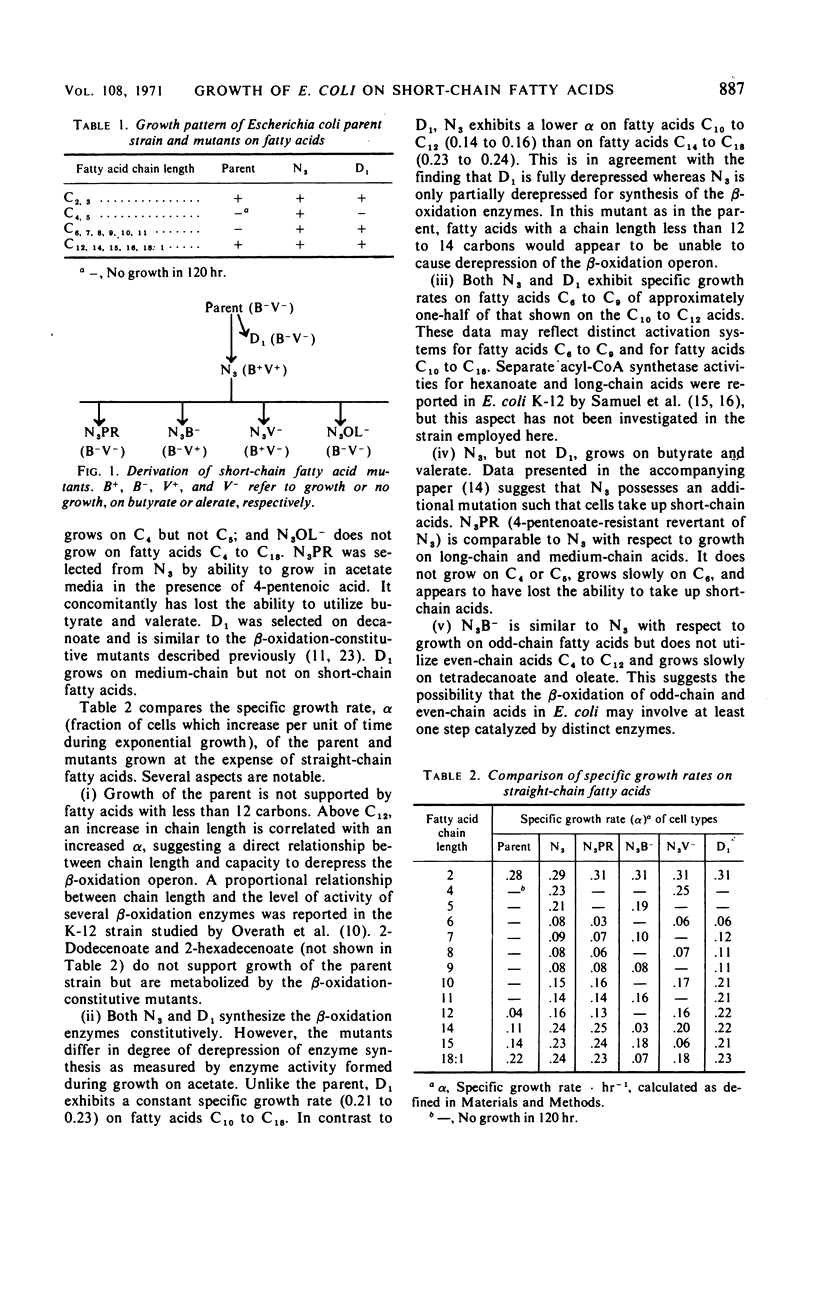
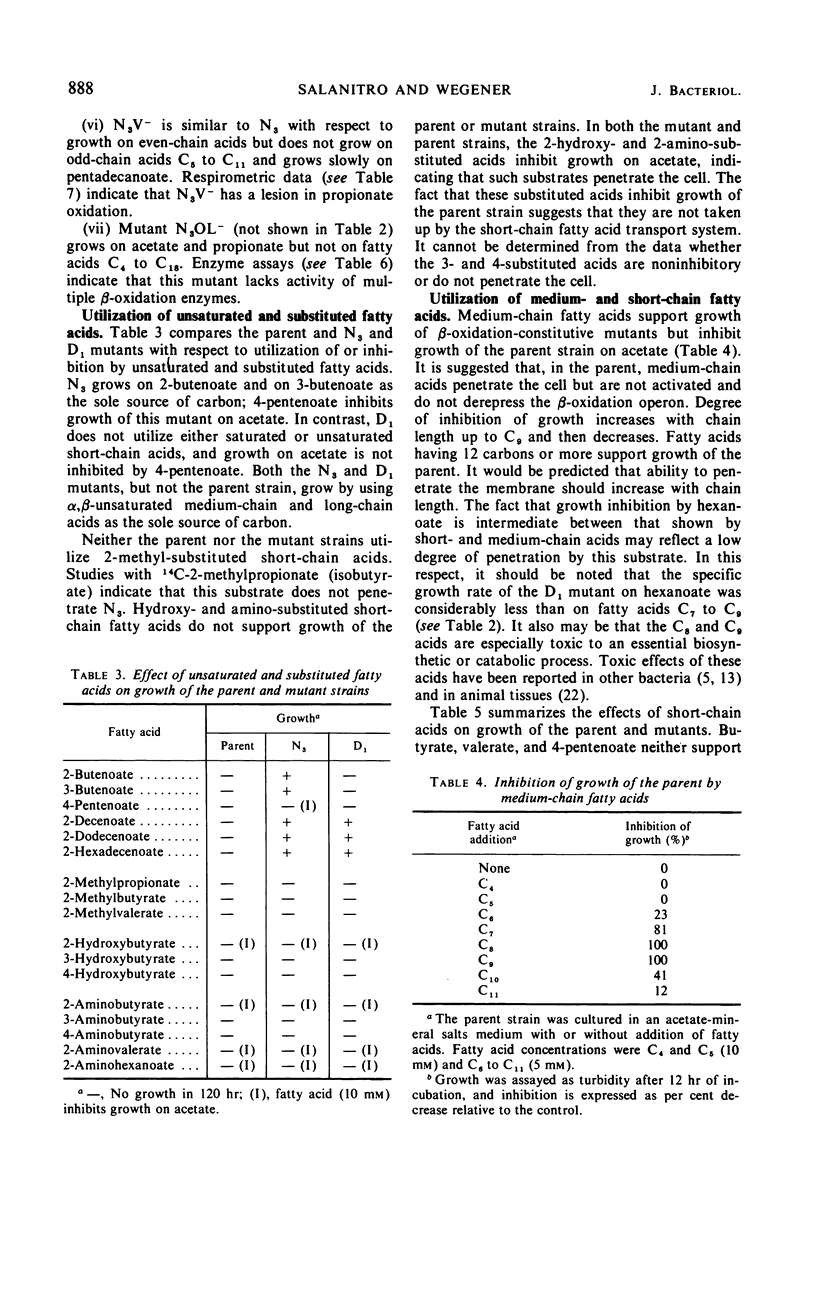
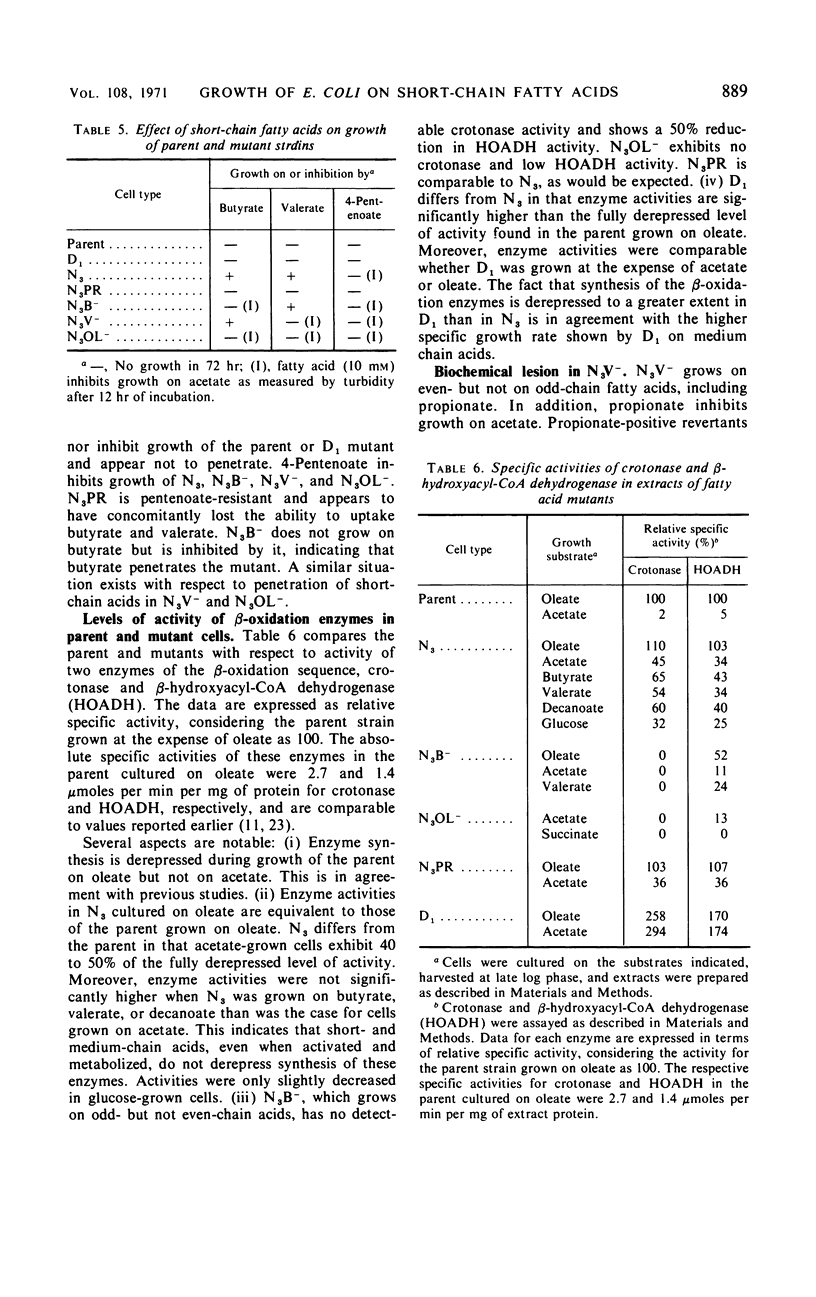
The parent Escherichia coli K-12 is constitutive for the enzymes of the glyoxylate bypass and adapts to growth on long-chain fatty acids (C12 to C18). It does not utilize medium-chain (C6 to C11) or short-chain (C4, C5) n-monocarboxylic acids. Several mutants of this strain which grow using short- or medium-chain acids, or both, as the sole carbon source were selected and characterized. One mutant (D1) synthesizes the β-oxidation enzymes constitutively and grows on medium-chain but not on short-chain acids. A second (N3) is partially derepressed for synthesis of these enzymes and grows both on medium-chain and on short-chain acids. Secondary mutants (N3V−, N3B−, N3OL−) were derived from N3. N3V− grows on even-chain but not on odd-chain acids and exhibits a lesion in propionate oxidation. N3B− grows on odd-chain but not on even-chain acids and exhibits no crotonase activity as assayed by hydration of crotonyl-CoA. N3OL− grows on acetate and propionate but does not utilize fatty acids C4 to C18; it exhibits multiple deficiencies in the β-oxidation pathway. Growth on acetate of N3, but not of the parent strain, is inhibited by 4-pentenoate. Revertants of N3 which are resistant to growth inhibition by 4-pentenoate (N3PR) exhibit loss of ability to grow on short-chain acids but retain the ability to grow on medium-chain and long-chain acids. The growth characteristics of these mutants suggest that in order to grow at the expense of butyrate and valerate, E. coli must be (i) derepressed for synthesis of the β-oxidation enzymes and (ii) derepressed for synthesis of a short-chain fatty acid uptake system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballard R. W., Palleroni N. J., Doudoroff M., Stanier R. Y., Mandel M. Taxonomy of the aerobic pseudomonads: Pseudomonas cepacia, P. marginata, P. alliicola and P. caryophylli. J Gen Microbiol. 1970 Feb;60(2):199–214. doi: 10.1099/00221287-60-2-199. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinandus J., Clark J. B. Selective inhibition of bacterial enzymes by free fatty acids. J Bacteriol. 1969 Jun;98(3):1109–1113. doi: 10.1128/jb.98.3.1109-1113.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis H. L., Johnson G. T. Intermediates of fatty acid metabolism by Cunninghamella echinulata. Mycologia. 1966 Jan-Feb;58(1):136–147. [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Palleroni N. J., Doudoroff M., Stanier R. Y., Solánes R. E., Mandel M. Taxonomy of the aerobic pseudomonads: the properties of the Pseudomonas stutzeri group. J Gen Microbiol. 1970 Feb;60(2):215–231. doi: 10.1099/00221287-60-2-215. [DOI] [PubMed] [Google Scholar]
- SILLIKER J. H., RITTENBERG S. C. Studies on the aerobic oxidation of fatty acids by bacteria, I. The nature of the enzymes, constitutive or adaptive. J Bacteriol. 1951 Jun;61(6):653–659. doi: 10.1128/jb.61.6.653-659.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILLIKER J. H., RITTENBERG S. C. Studies on the aerobic oxidation of fatty acids by bacteria. II. Application of the technique of simultaneous adaptation to the study of the mechanism of fatty acid oxidation in Serratia marcescens. J Bacteriol. 1951 Jun;61(6):661–673. doi: 10.1128/jb.61.6.661-673.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- Salanitro J. P., Wegener W. S. Growth of Escherichia coli on short-chain fatty acids: nature of the uptake system. J Bacteriol. 1971 Nov;108(2):893–901. doi: 10.1128/jb.108.2.893-901.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel D., Ailhaud G. Comparative aspects of fatty acid activation in Escherichia coli and Clostridium butyricum. FEBS Lett. 1969 Feb;2(4):213–216. doi: 10.1016/0014-5793(69)80022-0. [DOI] [PubMed] [Google Scholar]
- Samuel D., Estroumza J., Ailhaud G. Partial purification and properties of acyl-CoA synthetase of Escherichia coli. Eur J Biochem. 1970 Feb;12(3):576–582. doi: 10.1111/j.1432-1033.1970.tb00889.x. [DOI] [PubMed] [Google Scholar]
- Sartorelli L., Galzigna L., Rossi C. R., Gibson D. M. Influence of lecithin on the activity of the GTP-dependent acyl-coA synthetase. Biochem Biophys Res Commun. 1967 Jan 10;26(1):90–94. doi: 10.1016/0006-291x(67)90257-4. [DOI] [PubMed] [Google Scholar]
- Vanderwinkel E., Furmanski P., Reeves H. C., Ajl S. J. Growth of Escherichia coli on fatty acids: requirement for coenzyme A transferase activity. Biochem Biophys Res Commun. 1968 Dec 30;33(6):902–908. doi: 10.1016/0006-291x(68)90397-5. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., EVANS J. B., NIVEN C. J., Jr The oxidation of butyric acid by Streptococcus mitis. J Bacteriol. 1952 Oct;64(4):531–535. doi: 10.1128/jb.64.4.531-535.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Lea M. A., Stamm N. B. Regulation of hepatic carbohydrate metabolism by FFA and acetyl-CoA: sequential feedback inhibition. Lipids. 1969 Sep;4(5):388–396. doi: 10.1007/BF02531011. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. S., Furmanski P., Ajl S. J. Selection of mutants constitutive for several glyoxylate condensing enzymes during growth on valeric acid. Biochim Biophys Acta. 1967 Aug 8;144(1):34–50. [PubMed] [Google Scholar]
- Wegener W. S., Reeves H. C., Ajl S. J. Propionate oxidation in Escherichia coli. Arch Biochem Biophys. 1967 Aug;121(2):440–442. doi: 10.1016/0003-9861(67)90098-7. [DOI] [PubMed] [Google Scholar]
- Wegener W. S., Reeves H. C., Rabin R., Ajl S. J. Alternate pathways of metabolism of short-chain fatty acids. Bacteriol Rev. 1968 Mar;32(1):1–26. doi: 10.1128/br.32.1.1-26.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener W. S., Vanderwinkel E., Reeves H. C., Ajl S. J. Propionate metabolism. V. The physiological significance of isocitrate lyase during growth of E. coli on propionate. Arch Biochem Biophys. 1969 Feb;129(2):545–553. doi: 10.1016/0003-9861(69)90213-6. [DOI] [PubMed] [Google Scholar]



