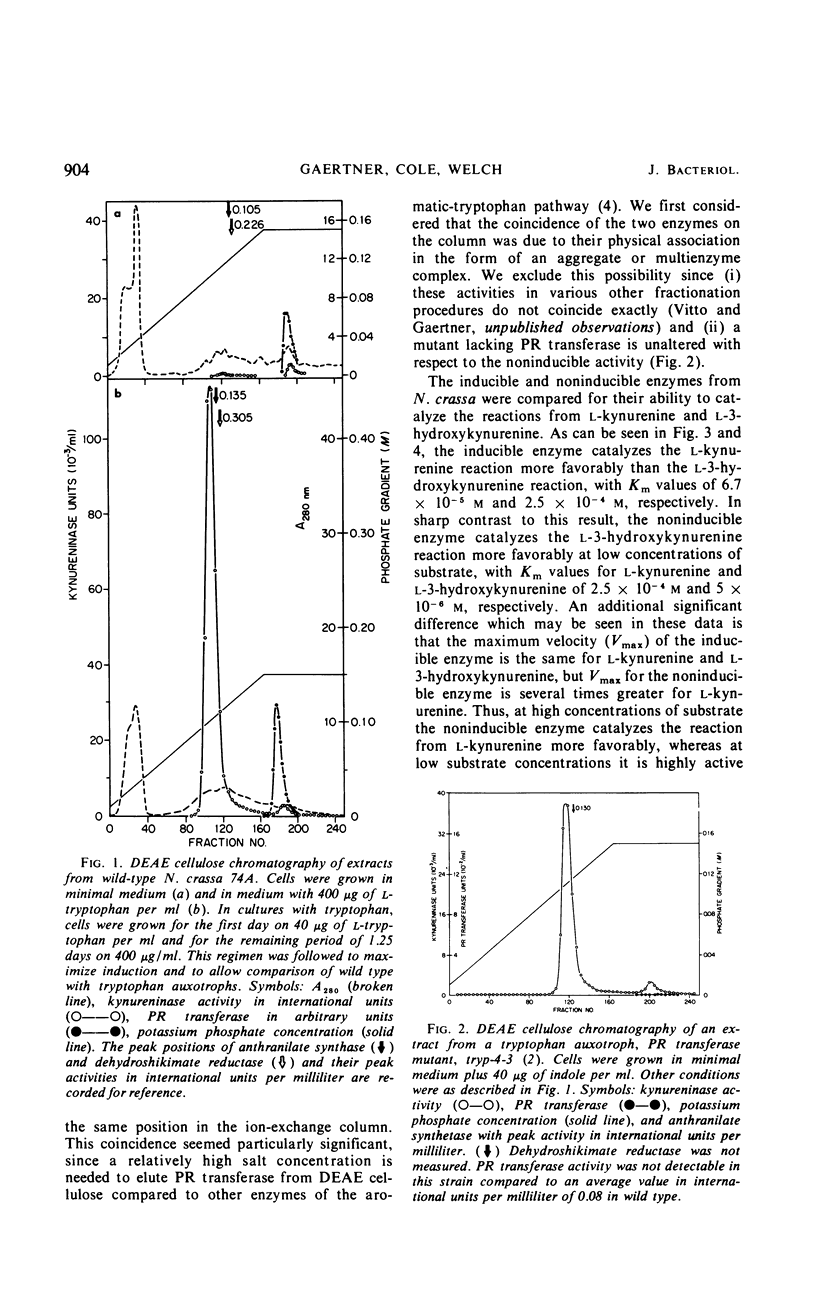
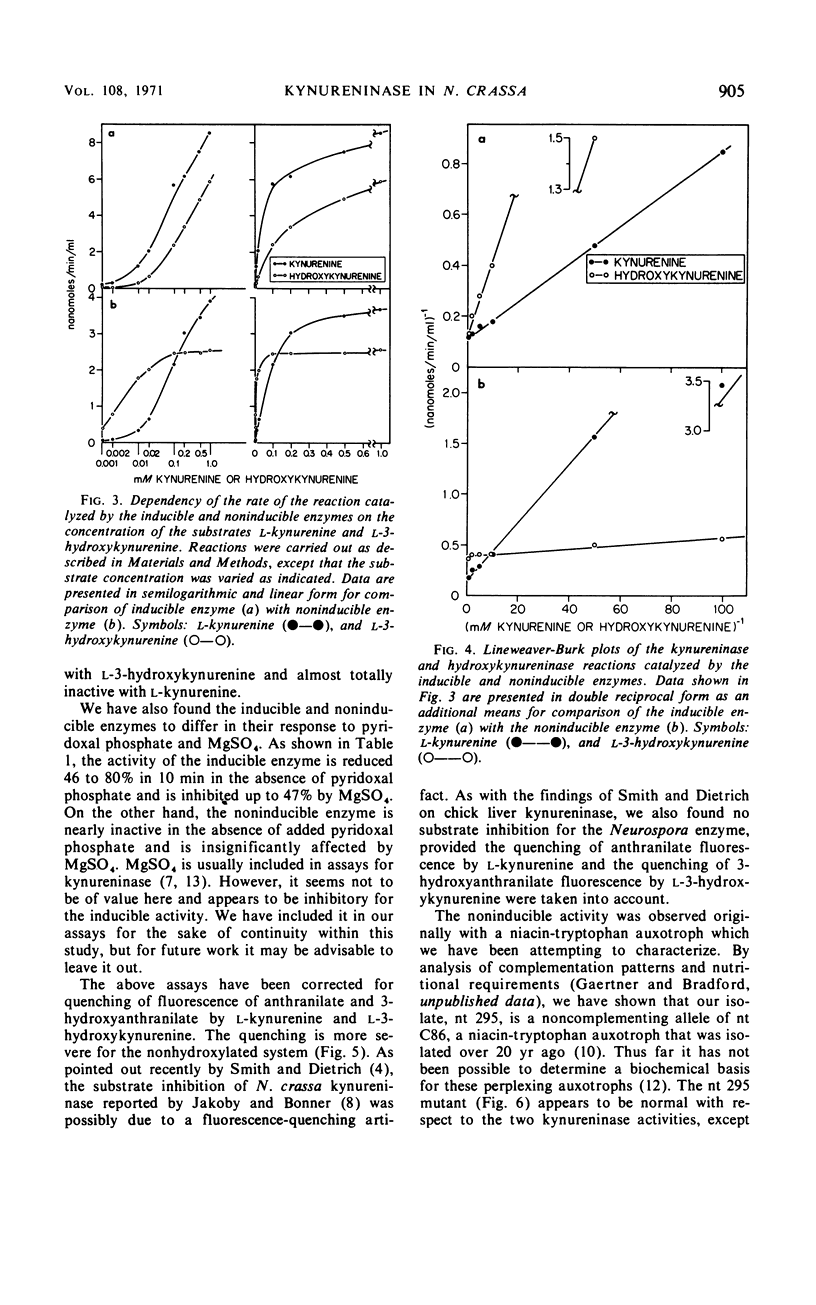
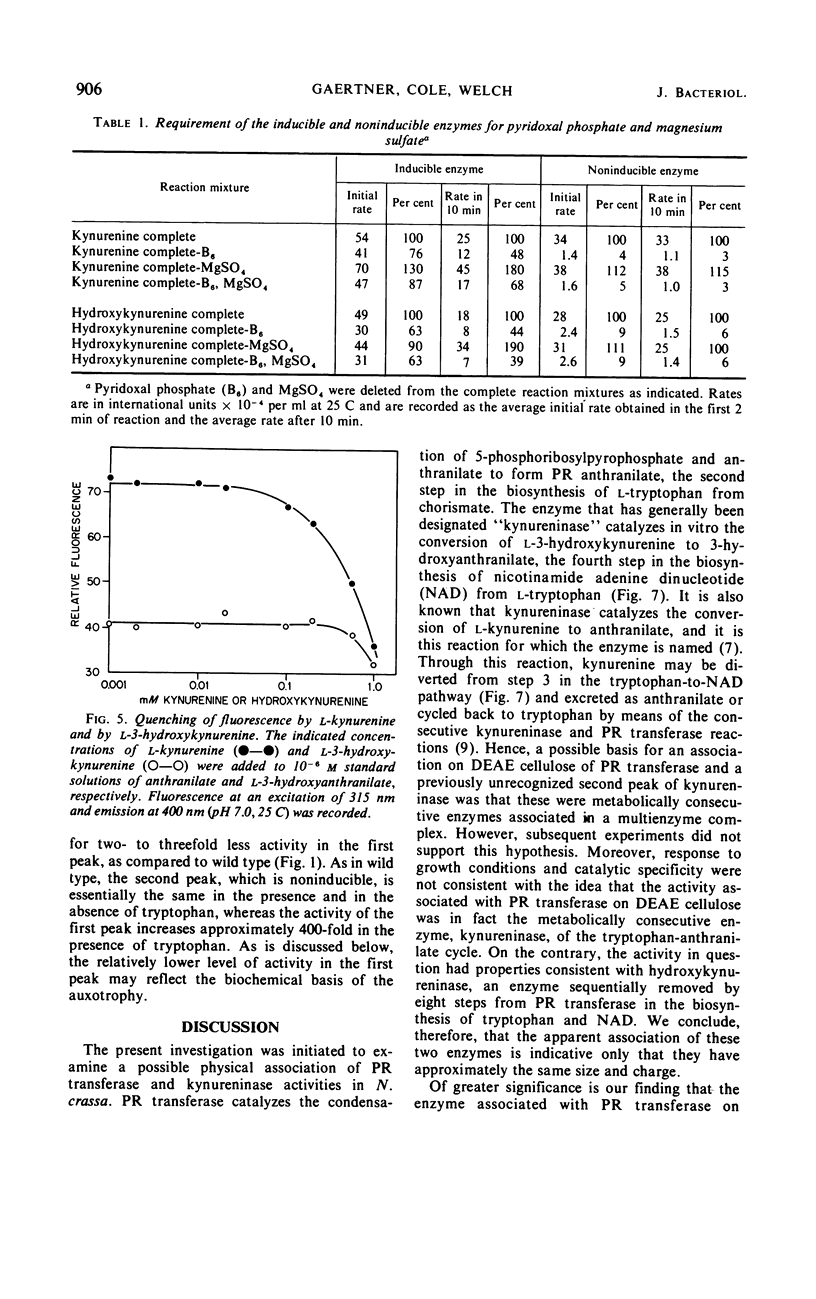
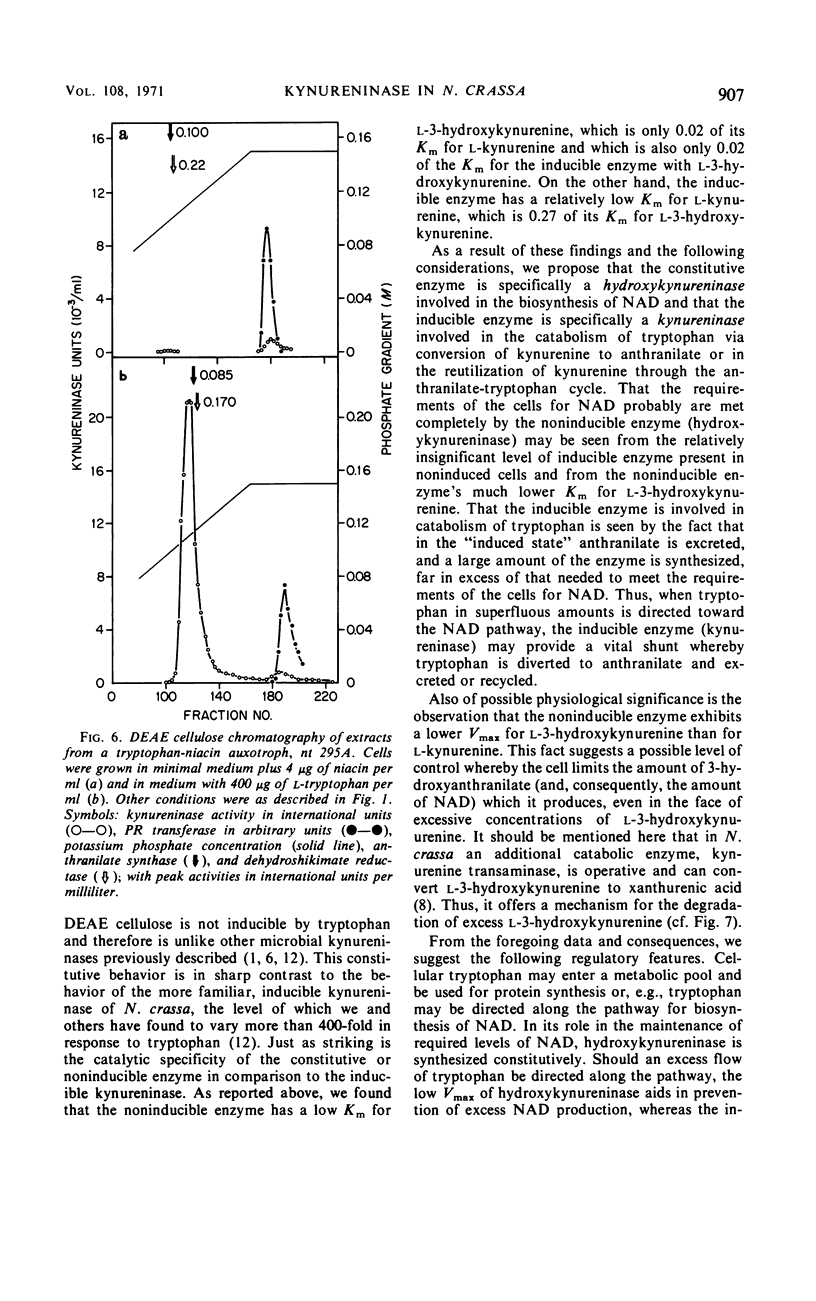
Abstract
Previous studies have indicated that a single enzyme, “kynureninase,” catalyzes the reactions of l-kynurenine to anthranilate and l-3-hydroxykynurenine to 3-hydroxyanthranilate in Neurospora crassa and in other organisms. The present report describes separate enzymes which catalyze these reactions in N. crassa. The first, a kynureninase, preferentially catalyzes kynurenine to anthranilate and is induced over 400-fold by tryptophan or a catabolite of tryptophan. The second, a hydroxykynureninase, is constitutive or noninducible by tryptophan and preferentially catalyzes l-3-hydroxykynurenine to 3-hydroxyanthranilate. The physiological significance of these enzymes may be inferred from the facts that (i) the noninducible enzyme hydroxykynureninase appears to be the main enzyme present in uninduced cells that is capable of catalyzing l-3-hydroxykynurenine to 3-hydroxyanthranilate for the indispensible synthesis of nicotinamide adenine dinucleotide, and (ii) the inducible enzyme kynureninase is induced by tryptophan to a concentration far in excess of that needed to meet the requirements of the cells for nicotinamide adenine dinucleotide, resulting in the excretion of anthranilate into the medium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONNER D. M., JAKOBY W. B. Kynurenine transaminase from neurospora. J Biol Chem. 1956 Aug;221(2):689–695. [PubMed] [Google Scholar]
- Brown A. T., Wagner C. Regulation of enzymes involved in the conversion of tryptophan to nicotinamide adenine dinucleotide in a colorless strain of Xanthomonas pruni. J Bacteriol. 1970 Feb;101(2):456–463. doi: 10.1128/jb.101.2.456-463.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A., Wegman J. An enzyme aggregate in the tryptophan pathway of Neurospora crassa. Proc Natl Acad Sci U S A. 1965 Jul;54(1):241–247. doi: 10.1073/pnas.54.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner F. H., DeMoss J. A. Purification and characterization of a multienzyme complex in the tryptophan pathway of Neurospora crassa. J Biol Chem. 1969 May 25;244(10):2716–2725. [PubMed] [Google Scholar]
- HAYAISHI O., STANIER R. Y. The kynureninase of Pseudomonas fluorescens. J Biol Chem. 1952 Apr;195(2):735–740. [PubMed] [Google Scholar]
- Haskins F. A., Mitchell H. K. Evidence for a Tryptophane Cycle in Neurospora. Proc Natl Acad Sci U S A. 1949 Sep;35(9):500–506. doi: 10.1073/pnas.35.9.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBY W. B., BONNER D. M. Kynureninase from Neurospora: purification and properties. J Biol Chem. 1953 Dec;205(2):699–707. [PubMed] [Google Scholar]
- MATCHETT W. H., DEMOSS J. A. Direct evidence for a trytophan-anthranilic acid cycle in Neurospora. Biochim Biophys Acta. 1963 Jun 4;71:632–642. doi: 10.1016/0006-3002(63)91136-3. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Dietrich L. S. Kynurenine metabolism in the developing chick embryo. Biochim Biophys Acta. 1971 Feb 23;230(2):271–278. doi: 10.1016/0304-4165(71)90213-3. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Drucker H. Kynureninase from Neurospora: occurrence of two activities. Biochem Biophys Res Commun. 1971 Feb 19;42(4):698–704. doi: 10.1016/0006-291x(71)90544-4. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Sorsoli W. A., Matchett W. H. Induction of kynureninase in Neurospora. J Bacteriol. 1970 Aug;103(2):364–369. doi: 10.1128/jb.103.2.364-369.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



