Abstract
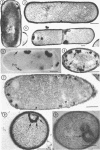
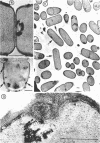
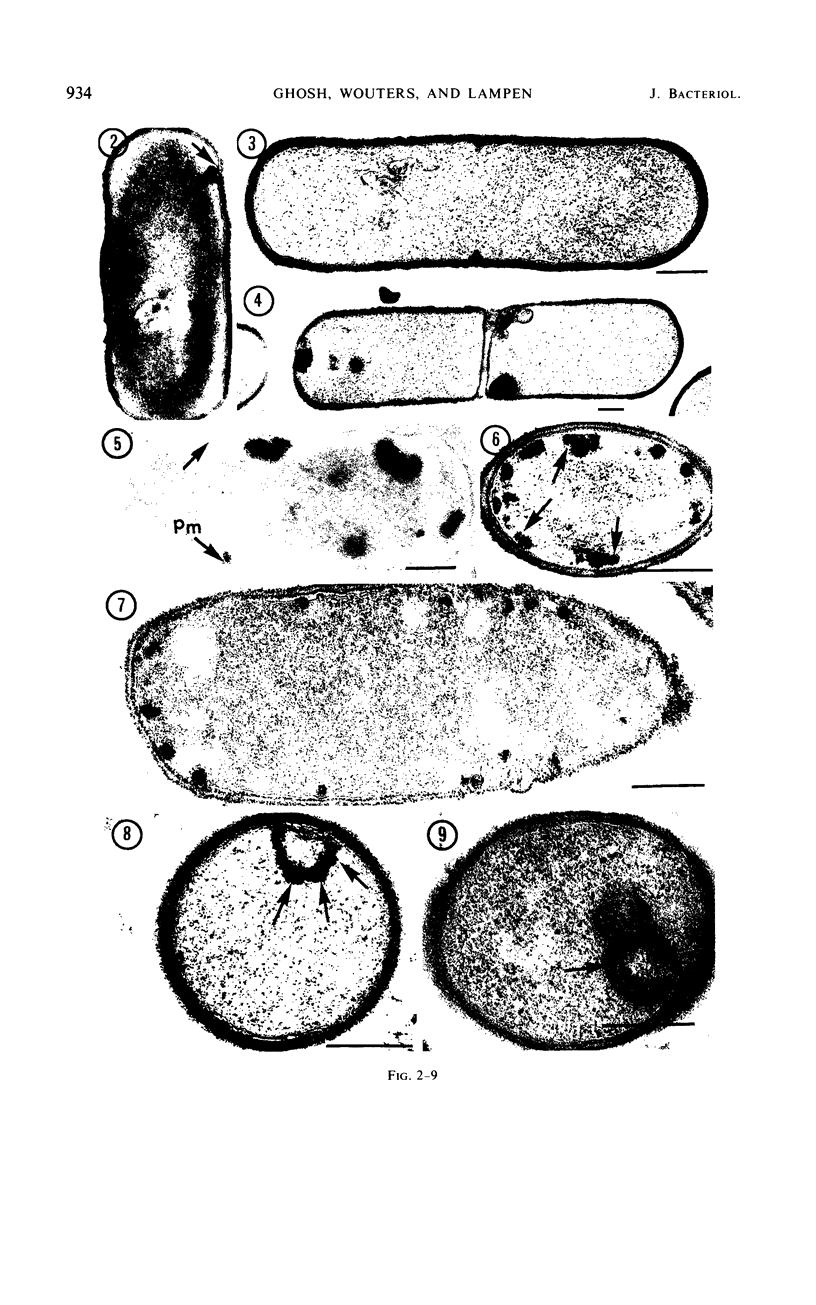
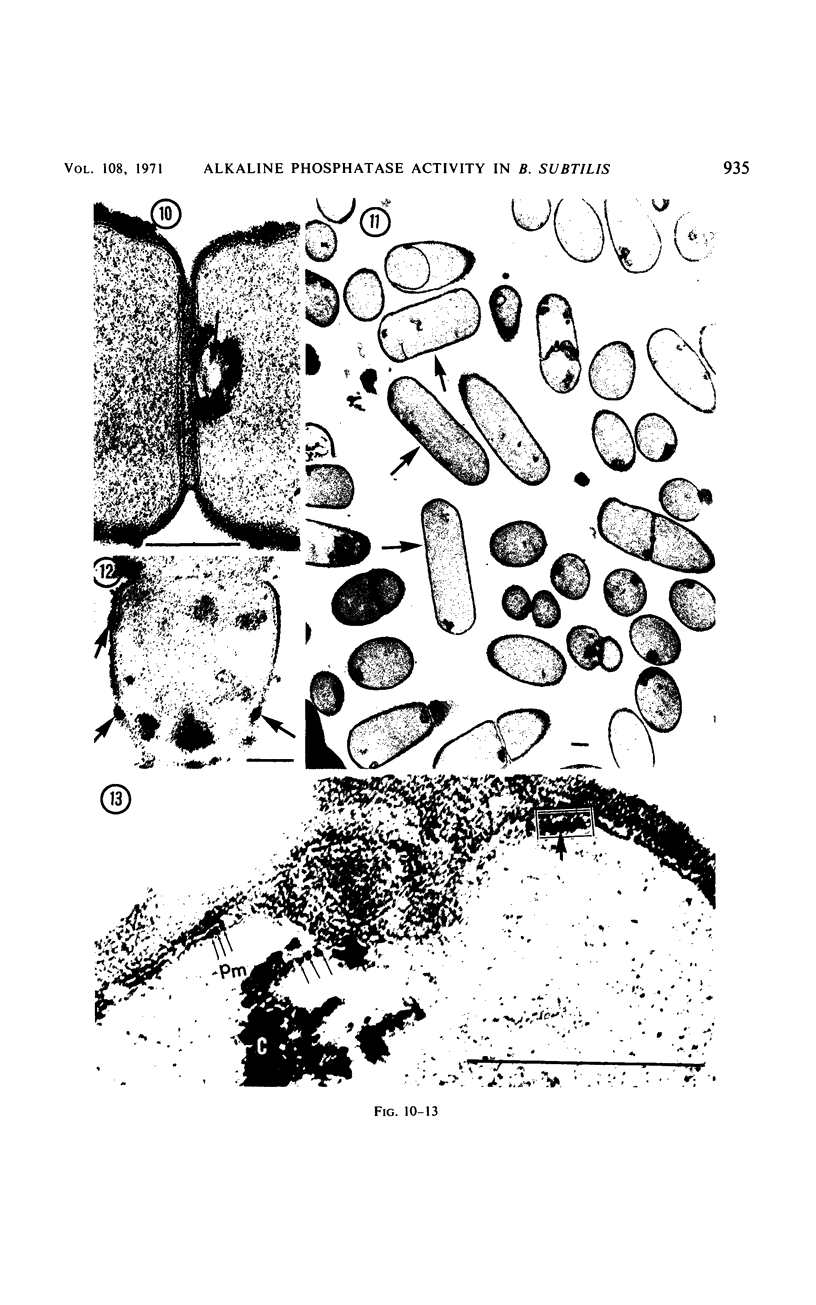
Sites of alkaline phosphatase activity have been located by an electron microscopic histochemical (Gomori) technique in vegetative cells of a repressible strain SB15 of Bacillus subtilis, derepressed and repressed by inorganic phosphate, and in a mutant SB1004 which forms alkaline phosphatase in a medium high in phosphate. The sites of enzyme activity were revealed as discrete, dense, and largely spherical bodies of varying sizes (20 to 150 nm). Cells of both repressible and repression-resistant strains acted on a wide variety of phosphate esters (p-nitrophenylphosphate, β-glycerophosphate, adenosine-5′-phosphate, glucose-6-phosphate, glucose-l-phosphate, adenosine triphosphate, and sodium pyrophosphate) to produce inorganic phosphorus under conditions of alkaline phosphatase assay [0.05 m tris(hydroxymethyl)aminomethane buffer (pH 8.4) containing 2 mm MgCl2]. The purified alkaline phosphatase also acted on all these esters, although much less effectively on adenosine triphosphate and sodium pyrophosphate than did the cells. Comparison of the relative utilization of the various substrates by repressed and derepressed cells and purified enzyme suggested the presence of multiple enzymes in the cells. Thus, the cytochemical method of trapping the newly generated inorganic phosphorus determines the location of an alkaline phosphatase of broad substrate profile, and in addition locates the sites of other enzymes generating inorganic phosphorus under identical conditions of assay. It is intriguing that all of these enzymes usually exist in a few clusters attached to the peripheral plasma membrane. In addition to this predominant location, there were a few sites of enzyme activity in the cytoplasm unattached to any discernible structure, and also in the cell wall of the repression-resistant and of the derepressed, repressible strains.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Dobozy A., Hammer H. Some properties of alkaline phosphatase in Bacillus species. Acta Microbiol Acad Sci Hung. 1969;16(2):181–187. [PubMed] [Google Scholar]
- Ghosh B. K., Sargent M. G., Lampen J. O. Morphological phenomena associated with penicillinase induction and secretion in Bacillus licheniformis. J Bacteriol. 1968 Oct;96(4):1314–1328. doi: 10.1128/jb.96.4.1314-1328.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnarev V. M., Smirnova T. A. Electron microscopy of alkaline phosphatase of Escherichia coli. Can J Microbiol. 1966 Aug;12(4):605–607. doi: 10.1139/m66-086. [DOI] [PubMed] [Google Scholar]
- MOELBERT E., DUSPIVA F., von DEIMLING [Histochemical localization of phosphatases in tubular epithelial cells of the mouse kidney in the electron microscopic picture]. Z Zellforch Microsk Anat Histochem. 1960;2:5–22. doi: 10.1007/BF00736487. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Rosenthal A. S. Pitfalls in the use of lead ion for histochemical localization of nucleoside phosphatases. J Histochem Cytochem. 1968 Aug;16(8):530–539. doi: 10.1177/16.8.530. [DOI] [PubMed] [Google Scholar]
- Munkres M., Wachtel A. Histochemical localization of phosphatases in Mycoplasma gallisepticum. J Bacteriol. 1967 Mar;93(3):1096–1103. doi: 10.1128/jb.93.3.1096-1103.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisonson I., Tannenbaum M., Neu H. C. Surface localization of Escherichia coli 5'-nucleotidase by electron microscopy. J Bacteriol. 1969 Nov;100(2):1083–1090. doi: 10.1128/jb.100.2.1083-1090.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Takeda K., Tsugita A. Phosphoesterases of Bacillus subtilis. II. Crystallization and properties of alkaline phosphatase. J Biochem. 1967 Feb;61(2):231–241. doi: 10.1093/oxfordjournals.jbchem.a128535. [DOI] [PubMed] [Google Scholar]
- VOELZ H. SITES OF ADENOSINE TRIPHOSPHATASE ACTIVITY IN BACTERIA. J Bacteriol. 1964 Oct;88:1196–1198. doi: 10.1128/jb.88.4.1196-1198.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz H., Ortigoza R. O. Cytochemistry of phosphatases in Myxococcus xanthus. J Bacteriol. 1968 Oct;96(4):1357–1365. doi: 10.1128/jb.96.4.1357-1365.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel B. K., Spicer S. S., Dvorak H. F., Heppel L. A. Cytochemical localization of certain phosphatases in Escherichia coli. J Bacteriol. 1970 Oct;104(1):529–542. doi: 10.1128/jb.104.1.529-542.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. A., Tristram H. Localization in the Cell and Extraction of Alkaline Phosphatase from Bacillus subtilis. J Bacteriol. 1970 Dec;104(3):1045–1051. doi: 10.1128/jb.104.3.1045-1051.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]