Abstract
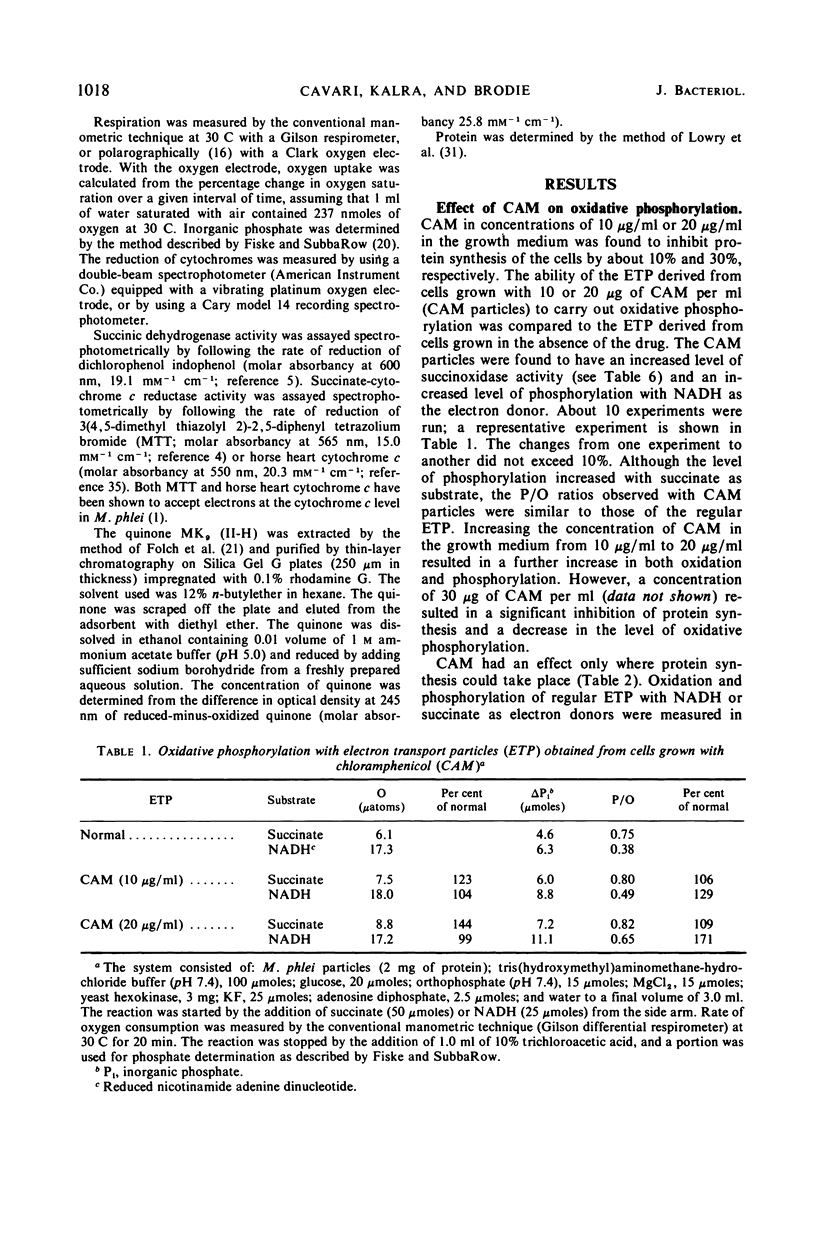
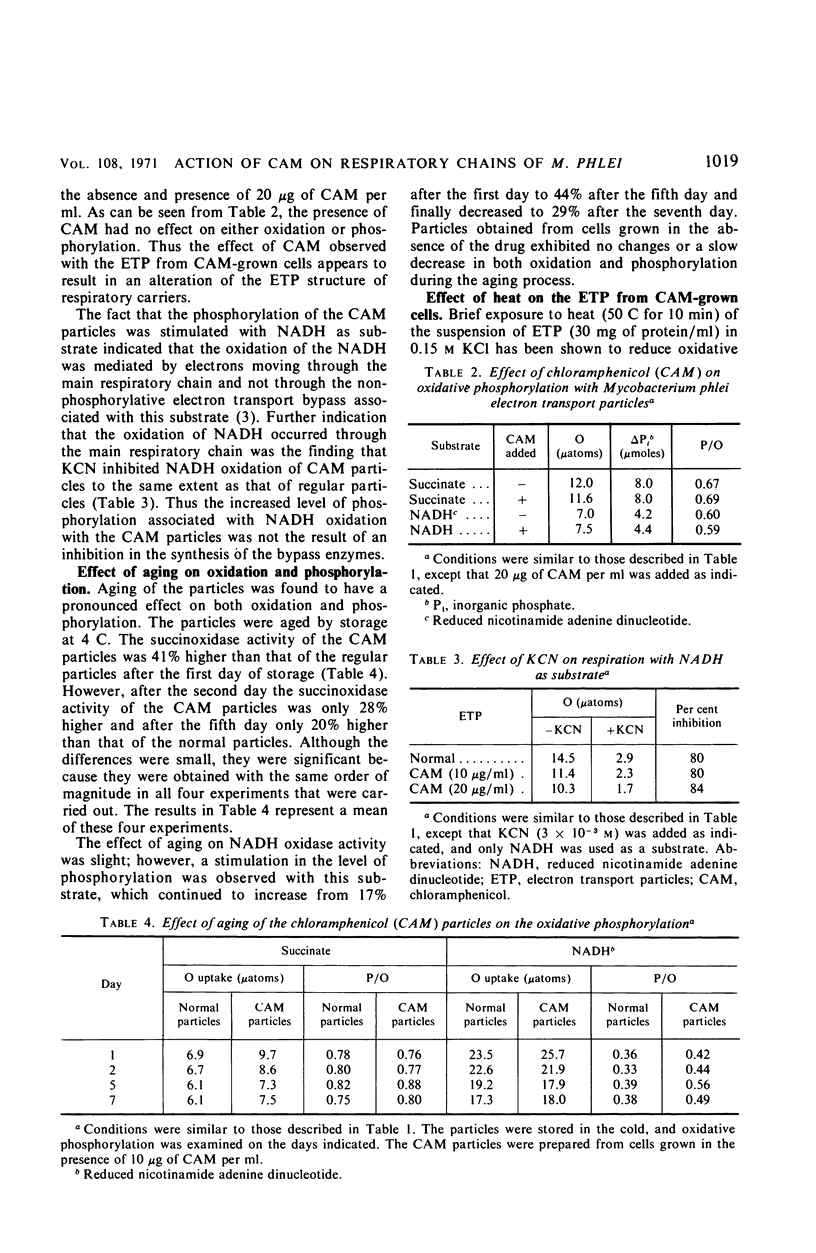
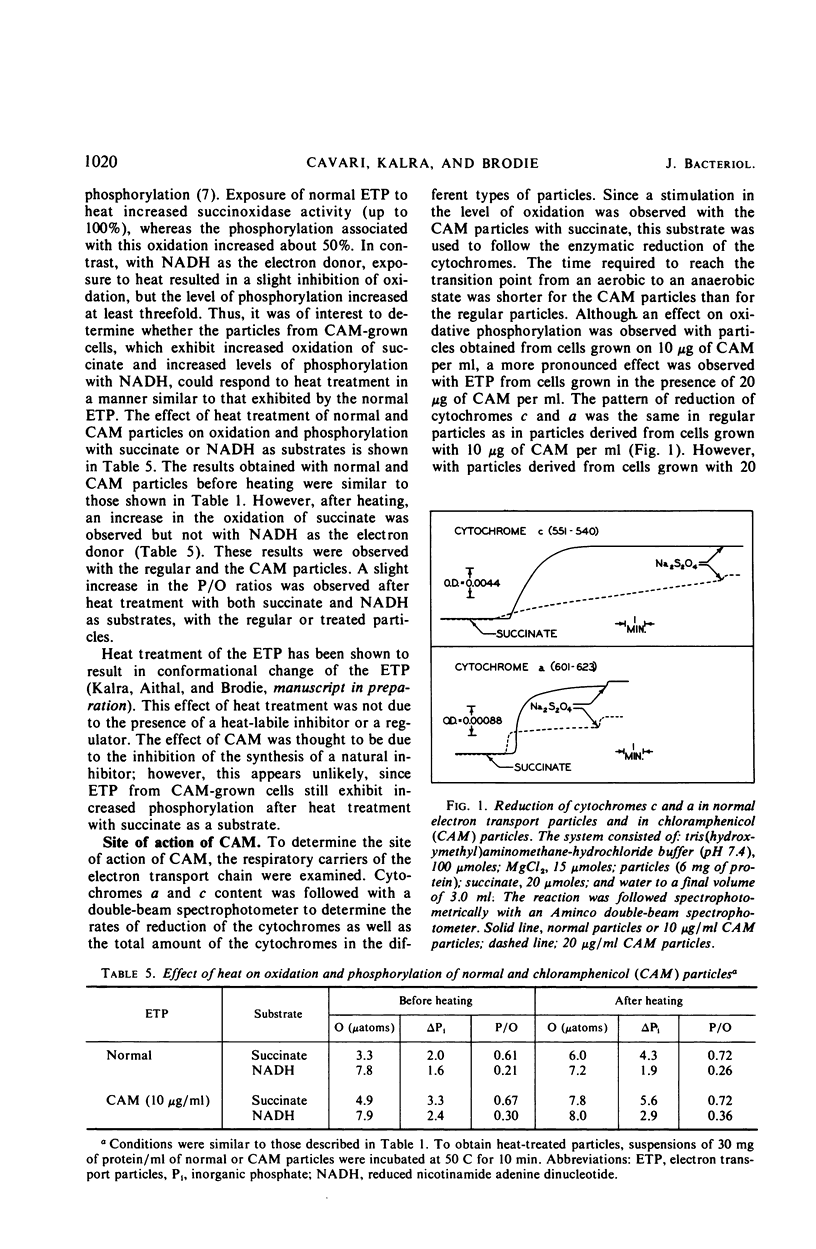
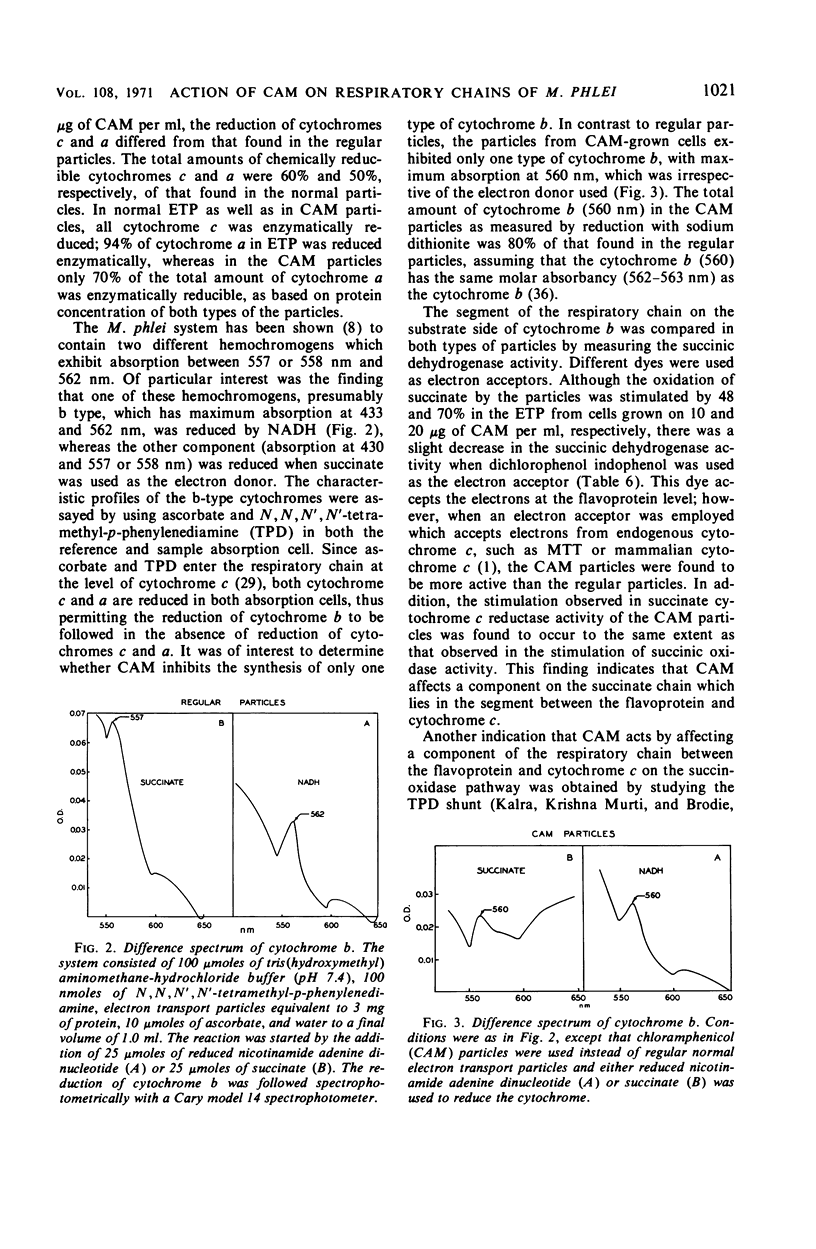
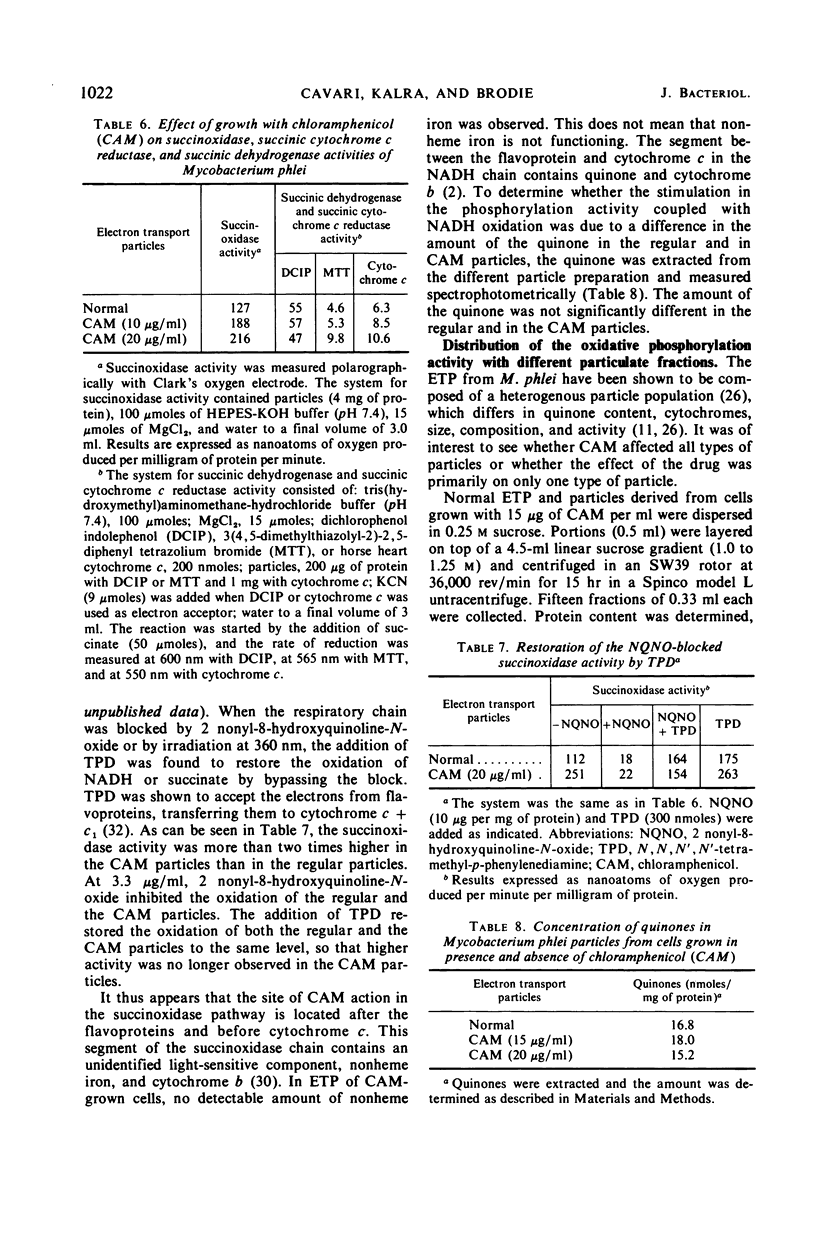
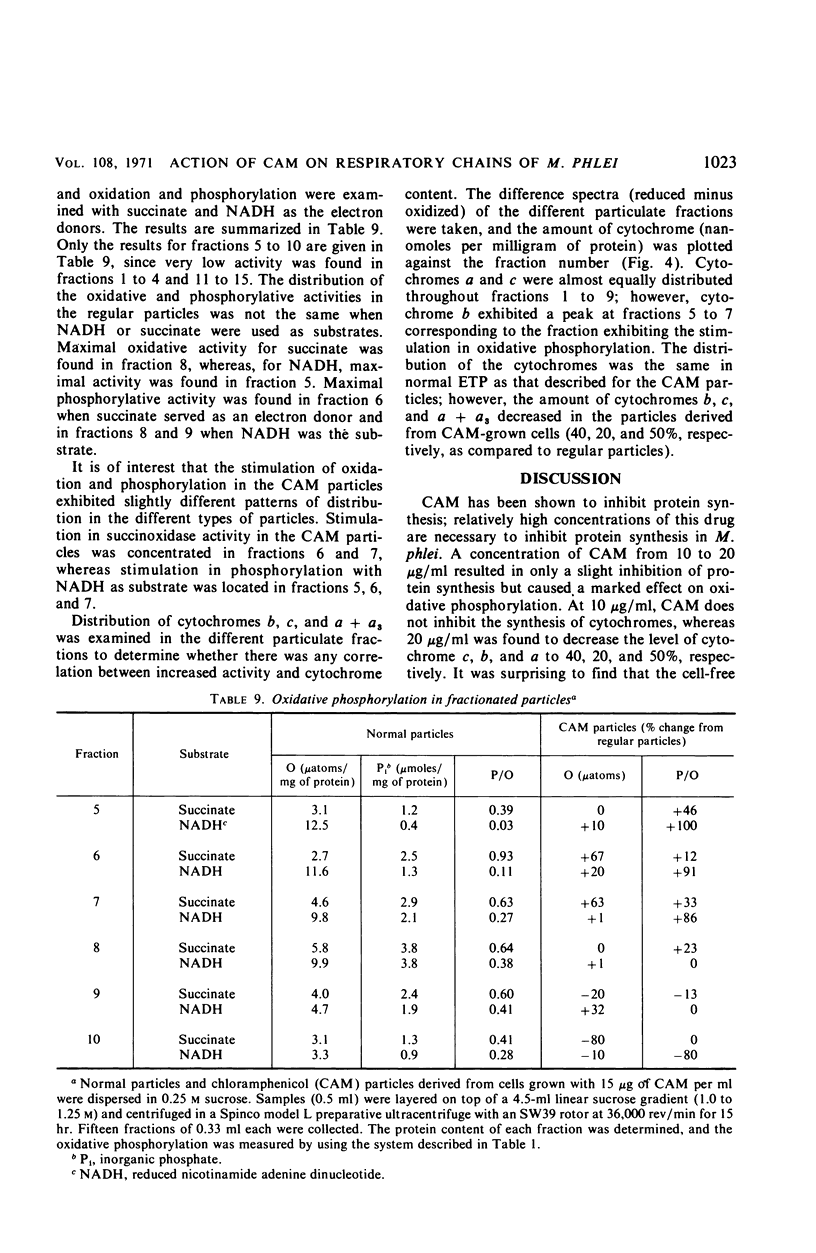
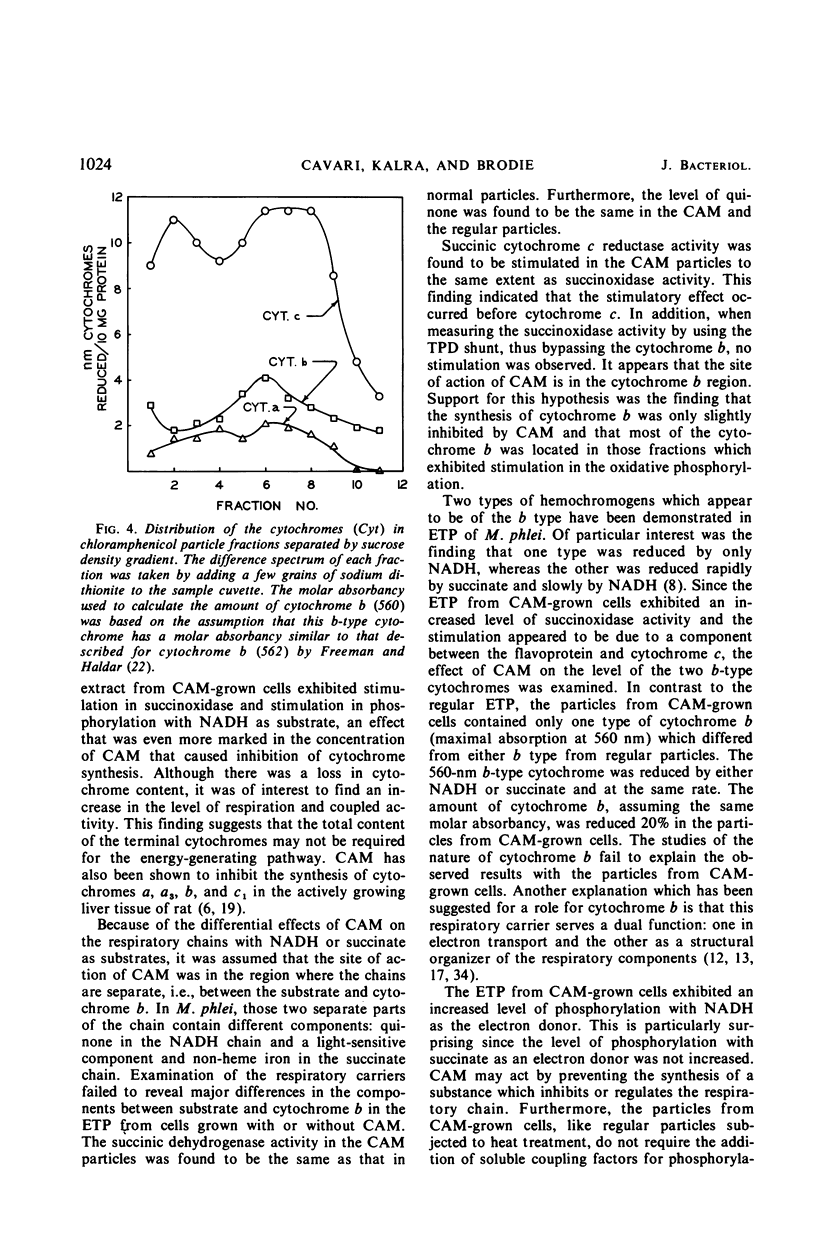
Chloramphenicol was found to have a direct effect on the respiratory chain of Mycobacterium phlei cells grown in the presence of this drug. Analysis of the respiratory chain components revealed that the presence of chloramphenicol during growth resulted in a partial inhibition in the synthesis of the cytochromes. However, a stimulation in oxidative phosphorylation was observed with the cell-free extract of cells grown in the presence of chloramphenicol. The oxidation of succinate was found to be stimulated 20 to 130%, depending on the particular extract, whereas the oxidation of reduced nicotinamide adenine dinucleotide (NADH) was found to be similar to that of extracts obtained from cells grown in the absence of the drug. Of particular interest was the finding that the cell-free extract of cells grown in the presence of the drug exhibited an increased level of phosphorylation (17 to 100%) when NADH was used as the electron donor. Chloramphenicol appears to affect a component of the respiratory chain between the flavoprotein and cytochrome c. Fractionation of the electron transport particles revealed an increased level of cytochrome b in the fractions which exhibited a stimulation in oxidative phosphorylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO A., BRODIE A. F. OXIDATIVE PHOSPHORYLATION IN FRACTIONATED BACTERIAL SYSTEMS. XIV. RESPIRATORY CHAINS OF MYCOBACTERIUM PHLEI. J Biol Chem. 1964 Dec;239:4280–4291. [PubMed] [Google Scholar]
- ASANO A., KANESHIRO T., BRODIE A. F. MALATE-VITAMIN K REDUCTASE, A PHOSPHOLIPID-REQUIRING ENZYME. J Biol Chem. 1965 Feb;240:895–905. [PubMed] [Google Scholar]
- Asano A., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. 18. Phosphorylation coupled to different segments of the respiratory chains of Mycobacterium phlei. J Biol Chem. 1965 Oct;240(10):4002–4010. [PubMed] [Google Scholar]
- BRODIE A. F., GRAY C. T. Phosphorylation coupled to oxidation in bacterial extracts. J Biol Chem. 1956 Apr;219(2):853–862. [PubMed] [Google Scholar]
- Beattie D. S. Studies on the biogenesis of mitochondrial protein components in rat liver slices. J Biol Chem. 1968 Aug 10;243(15):4027–4033. [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. F. Effects of heat treatment of electron-transport particles on bacterial oxidative phosphorylation. Proc Natl Acad Sci U S A. 1970 Sep;67(1):1–6. doi: 10.1073/pnas.67.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin E., Higashi T., Brodie A. Extraparticulate chain interaction between different electron transport particles. Science. 1969 Sep 26;165(3900):1364–1367. doi: 10.1126/science.165.3900.1364. [DOI] [PubMed] [Google Scholar]
- Brock T. D. CHLORAMPHENICOL. Bacteriol Rev. 1961 Mar;25(1):32–48. doi: 10.1128/br.25.1.32-48.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie A. F., Revsin B., Kalra V., Phillips P., Bogin E., Higashi T., Murti C. R., Cavari B. Z., Marquez E. Biological function of terpenoid guinones. Biochem Soc Symp. 1970;29:119–143. [PubMed] [Google Scholar]
- Bruni A., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. I. Reconstitution of the succinate-ubiquinone reductase. J Biol Chem. 1968 Mar 10;243(5):962–971. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R., HOLMES W. F., HIGGINS J. Respiratory enzymes in oxidative phosphorylation. V. A mechanism for oxidative phosphorylation. J Biol Chem. 1955 Nov;217(1):439–451. [PubMed] [Google Scholar]
- CLARK L. C., Jr, WOLF R., GRANGER D., TAYLOR Z. Continuous recording of blood oxygen tensions by polarography. J Appl Physiol. 1953 Sep;6(3):189–193. doi: 10.1152/jappl.1953.6.3.189. [DOI] [PubMed] [Google Scholar]
- Chance B., Schoener B. High and low energy states of cytochromes. I. In mitochondria. J Biol Chem. 1966 Oct 25;241(20):4567–4573. [PubMed] [Google Scholar]
- Clark-Walker G. D., Linnane A. W. The biogenesis of mitochondria in Saccharomyces cerevisiae. A comparison between cytoplasmic respiratory-deficient mutant yeast and chlormaphenicol-inhibited wild type cells. J Cell Biol. 1967 Jul;34(1):1–14. doi: 10.1083/jcb.34.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A. P., Cox G. F., Selwyn M. J. An effect of malate on the redox state of a cytochrome B component in mitochondria from various sources. Biochem Biophys Res Commun. 1968 Aug 21;32(4):579–587. doi: 10.1016/0006-291x(68)90276-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., ASCOLI I., LEES M., MEATH J. A., LeBARON N. Preparation of lipide extracts from brain tissue. J Biol Chem. 1951 Aug;191(2):833–841. [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Biogenesis of mitochondria. 8. The effect of chloramphenicol on regenerating rat liver. Exp Cell Res. 1969 Apr;55(1):68–76. doi: 10.1016/0014-4827(69)90457-1. [DOI] [PubMed] [Google Scholar]
- Firkin F. C., Linnane A. W. Differential effects of chloramphenicol on the growth and respiration of mammalian cells. Biochem Biophys Res Commun. 1968 Aug 13;32(3):398–402. doi: 10.1016/0006-291x(68)90674-8. [DOI] [PubMed] [Google Scholar]
- Freeman K. B., Haldar D. The inhibition of NADH oxidation in mammalian mitochondria by chloramphenicol. Biochem Biophys Res Commun. 1967 Jul 10;28(1):8–12. doi: 10.1016/0006-291x(67)90397-x. [DOI] [PubMed] [Google Scholar]
- HANSON J. B., HODGES T. K. UNCOUPLING ACTION OF CHLORAMPHENICOL AS A BASIS FOR THE INHIBITION OF ION ACCUMULATION. Nature. 1963 Dec 7;200:1009–1009. doi: 10.1038/2001009a0. [DOI] [PubMed] [Google Scholar]
- Higashi T., Bogin E., Brodie A. F. Separation of a factor indispensable for coupled phosphorylation from the particulate fraction of Mycobacterium phlei. J Biol Chem. 1969 Jan 25;244(2):500–502. [PubMed] [Google Scholar]
- Horstman L. L., Racker E. Partial resolution of the enzyme catalyzing oxidative phosphorylation. XXII. Interaction between mitochondrial adenosine triphosphatase inhibitor and mitochondrial adenosine triphosphatase. J Biol Chem. 1970 Mar 25;245(6):1336–1344. [PubMed] [Google Scholar]
- Huang M., Biggs D. R., Clark-Walker G. D., Linnane A. W. Chloramphenicol inhibition of the formation of particulate mitochondrial enzymes of Saccharomyces cerevisiae. Biochim Biophys Acta. 1966 Feb 21;114(2):434–436. doi: 10.1016/0005-2787(66)90330-3. [DOI] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. Phosphorylation coupled to electron transport mediated by high potential electron carriers. Biochim Biophys Acta. 1960 Feb 12;38:12–34. doi: 10.1016/0006-3002(60)91192-6. [DOI] [PubMed] [Google Scholar]
- Kurup C. K., Brodie A. F. Oxidative phosphorylation in fractionated bacterial systems. XXV. Studies on the involvement of metal in Mycobacterium phlei. J Biol Chem. 1967 Jan 25;242(2):197–203. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mustafa M. G., Cowger M. L., Labbe R. F., King T. E. General nature of "Wurster's blue shunts" in the respiratory chain. J Biol Chem. 1968 Apr 25;243(8):1908–1918. [PubMed] [Google Scholar]
- PULLMAN M. E., MONROY G. C. A NATURALLY OCCURRING INHIBITOR OF MITOCHONDRIAL ADENOSINE TRIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3762–3769. [PubMed] [Google Scholar]
- YLER D. D., ESTABROOK R. W., SANADI D. R. ELECTRON AND ENERGY REQUIREMENTS FOR CYTOCHROME B REDUCTION DURING THE OXIDATION OF TETRAMETHYL-P-PHENYLENE DIAMINE. Biochem Biophys Res Commun. 1965 Jan 18;18:264–269. doi: 10.1016/0006-291x(65)90751-5. [DOI] [PubMed] [Google Scholar]
- ZAUGG W. S., RIESKE J. S. The quantitative estimation of cytochrome b in sub-mitochondrial particles from beef heart. Biochem Biophys Res Commun. 1962 Oct 17;9:213–217. doi: 10.1016/0006-291x(62)90060-8. [DOI] [PubMed] [Google Scholar]
- van GELDER B., SLATER E. C. The extinction coefficient of cytochrome c. Biochim Biophys Acta. 1962 Apr 23;58:593–595. doi: 10.1016/0006-3002(62)90073-2. [DOI] [PubMed] [Google Scholar]



