Abstract
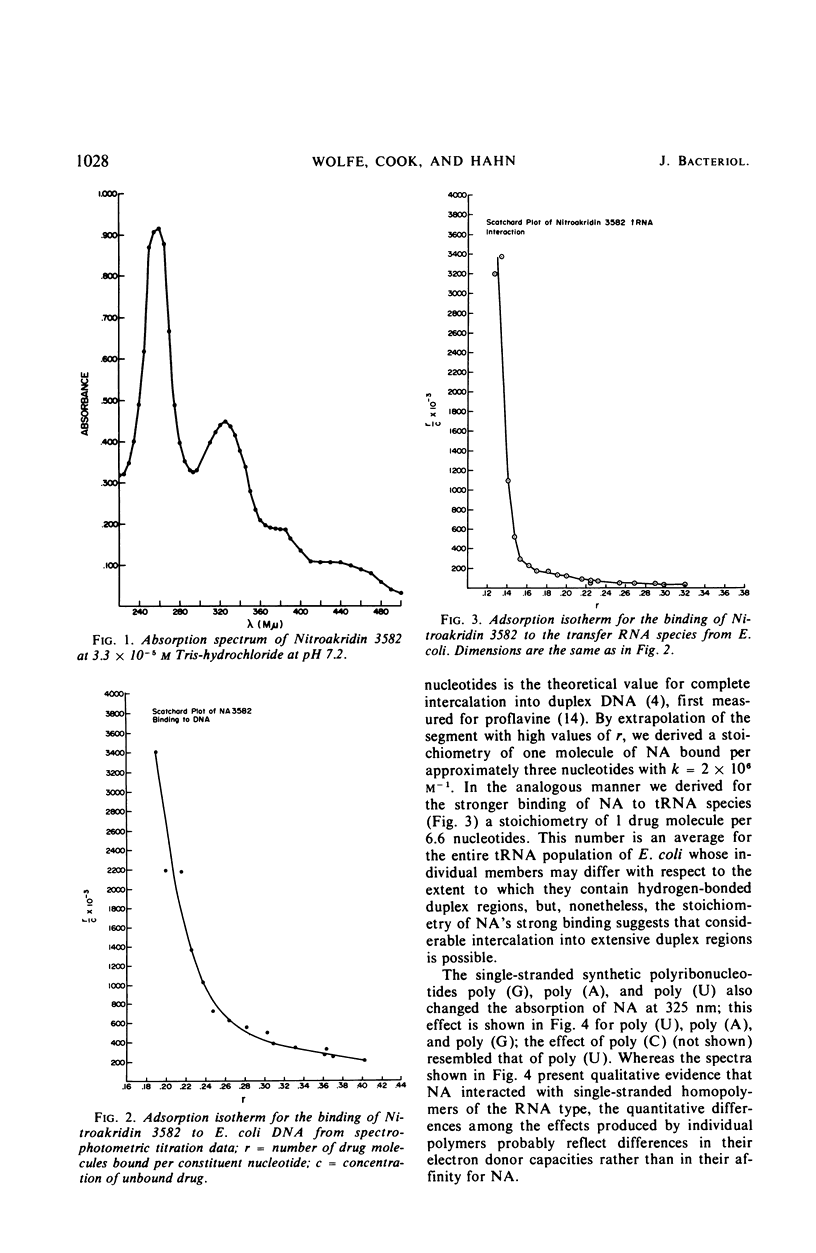
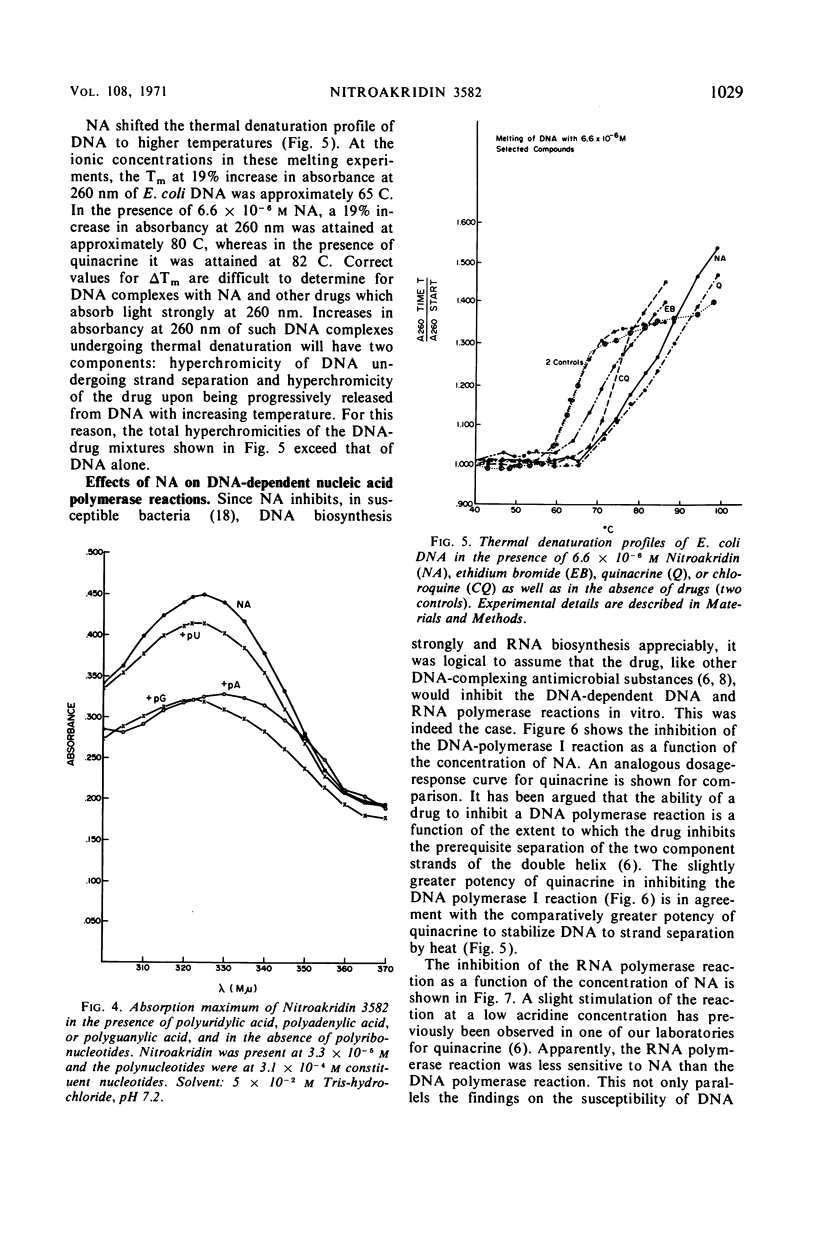
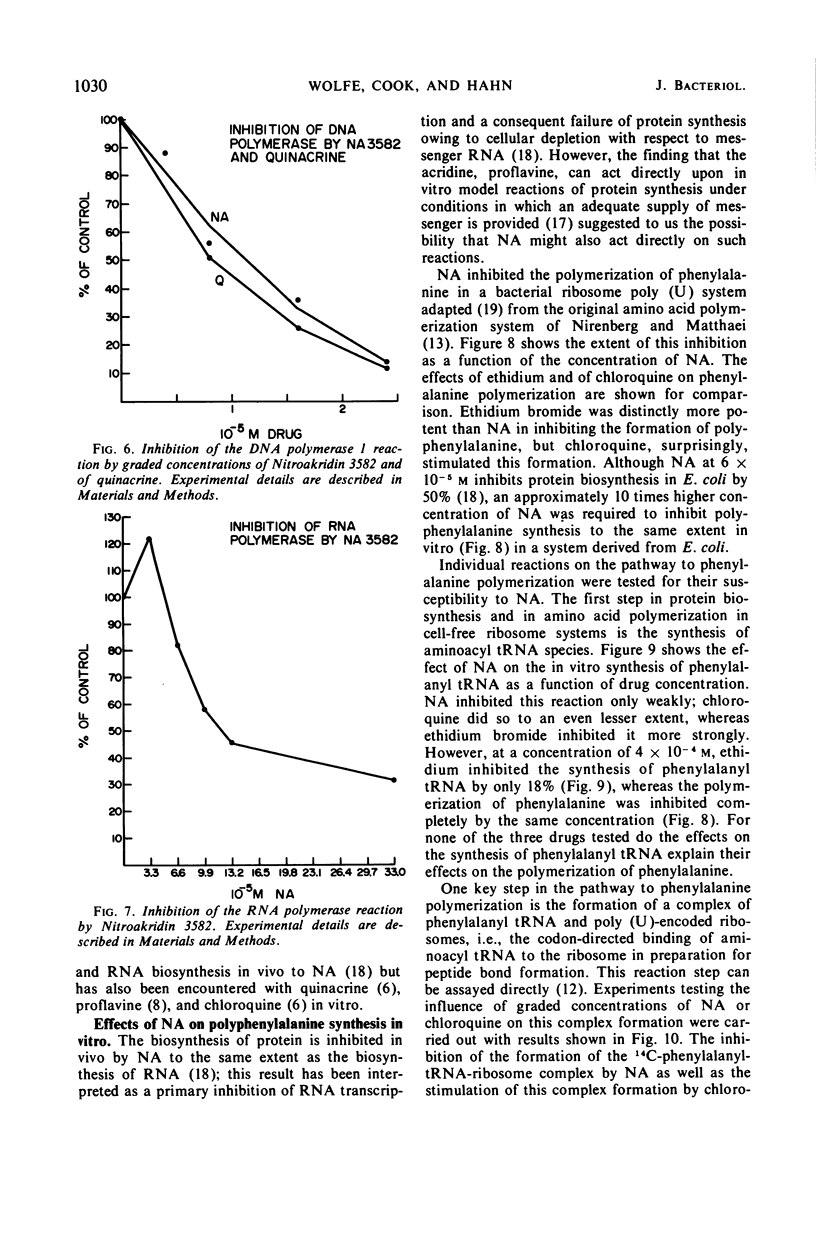
Nitroakridin 3582 (NA) formed complexes with native deoxyribonucleic acid (DNA) and with transfer ribonucleic acid (tRNA) species from Escherichia coli. Spectrophotometric titrations of NA with these nucleic acids produced numerical results from which nonlinear adsorption isotherms were derived. These curves indicated the existence of more than one class of binding sites on the polymers to which NA was bound by more than one process. The stoichiometry of strong binding of NA to double helical DNA was in agreement with a conventional value (1 ligand molecule per 4.2 component nucleotides) for complete intercalation binding. NA inhibited the DNA-dependent DNA polymerase I and RNA polymerase reactions, the first strongly and the second appreciably. These inhibitions corresponded to the extents to which NA inhibits DNA and RNA biosyntheses in vivo. Evidently, NA interferes with the template function of DNA. The drug also inhibited the polymerization of phenylalanine in a cell-free E. coli ribosome-polyuridylic acid [poly (U)] system. The effect paralleled an inhibition of the poly (U)-directed binding of phenylalanyl tRNA to ribosomes. Ethidium bromide acted similarly. The antimalarial drug, chloroquine, stimulated polyphenylalanine synthesis, apparently as a result of stimulating the poly (U)-directed binding of phenylalanyl tRNA to ribosomes.
Full text
PDF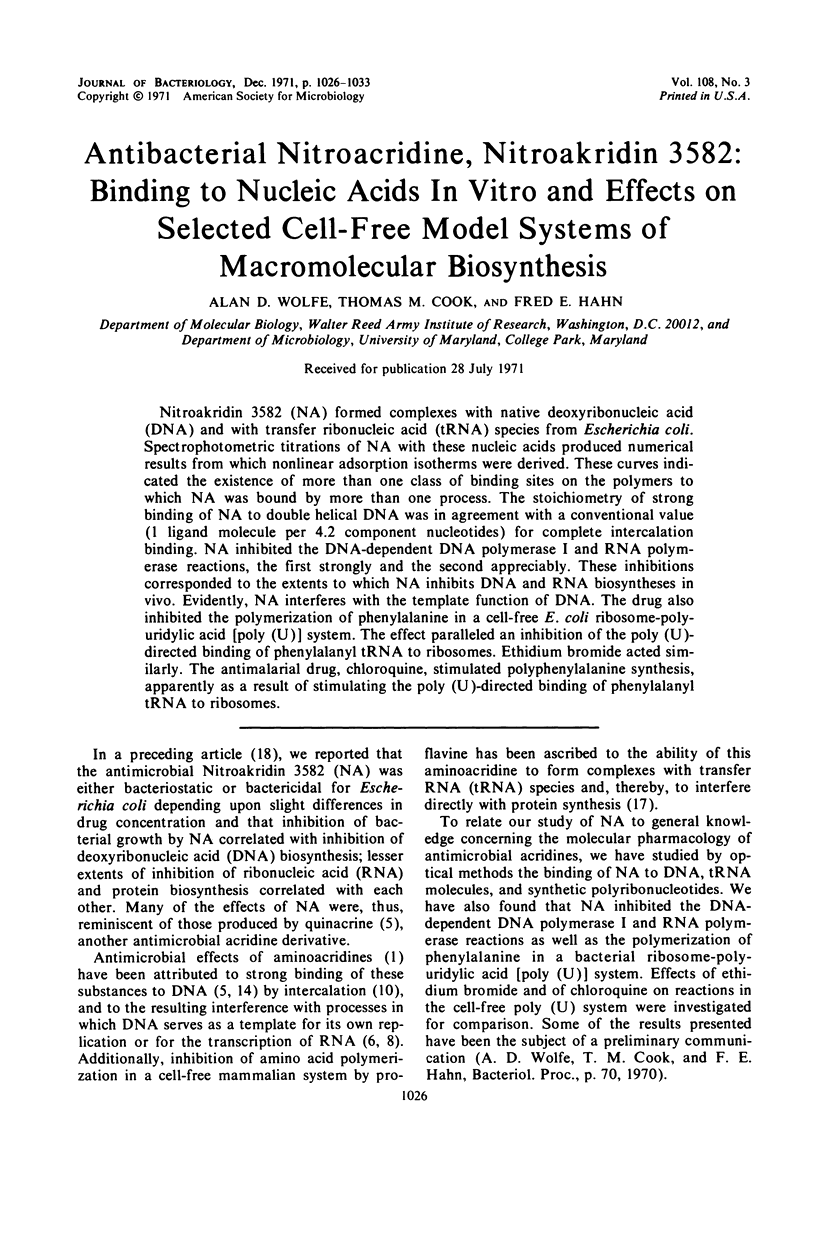



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittman R. Studies of the binding of ethidium bromide to transfer ribonucleic acid: absorption, fluorescence, ultracentrifugation and kinetic investigations. J Mol Biol. 1969 Dec 14;46(2):251–268. doi: 10.1016/0022-2836(69)90420-3. [DOI] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. The interaction of aminocridines with nucleic acids. Biopolymers. 1968;6(9):1225–1253. doi: 10.1002/bip.1968.360060902. [DOI] [PubMed] [Google Scholar]
- CAIRNS J. The application of autoradiography to the study of DNA viruses. Cold Spring Harb Symp Quant Biol. 1962;27:311–318. doi: 10.1101/sqb.1962.027.001.029. [DOI] [PubMed] [Google Scholar]
- Ciak J., Hahn F. E. Quinacrine (atebrin): mode of action. Science. 1967 May 5;156(3775):655–656. doi: 10.1126/science.156.3775.655. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Yielding K. L. Inhibition of DNA and RNA polymerase reactions by chloroquine. Proc Natl Acad Sci U S A. 1965 Aug;54(2):521–527. doi: 10.1073/pnas.54.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABOUREL J. D. Effects of hydroxychloroquine on the growth of mammalian cells in vitro. J Pharmacol Exp Ther. 1963 Jul;141:122–130. [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURNICK N. B., RADCLIFFE I. E. Reaction between DNA and quinacrine and other antimalarials. J Lab Clin Med. 1962 Oct;60:669–688. [PubMed] [Google Scholar]
- LERMAN L. S. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961 Feb;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- Muench K. H. Chloroquine-mediated conversion of transfer ribonucleic acid of Escherichia coli from an inactive to an active state. Cold Spring Harb Symp Quant Biol. 1966;31:539–542. doi: 10.1101/sqb.1966.031.01.069. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Polet H., Barr C. F. Chloroquine and dihydroquinine. In vitro studies by their antimalarial effect upon Plasmodium knowlesi. J Pharmacol Exp Ther. 1968 Dec;164(2):380–386. [PubMed] [Google Scholar]
- WOLFE A. D., HAHN F. E. MODE OF ACTION OF CHLORAMPHENICOL. IX. EFFECTS OF CHLORAMPHENICOL UPON A RIBOSOMAL AMINO ACID POLYMERIZATION SYSTEM AND ITS BINDING TO BACTERIAL RIBOSOME. Biochim Biophys Acta. 1965 Jan 11;95:146–155. doi: 10.1016/0005-2787(65)90219-4. [DOI] [PubMed] [Google Scholar]
- Weinstein I. B., Finkelstein I. H. Proflavine inhibition of protein synthesis. J Biol Chem. 1967 Sep 10;242(17):3757–3762. [PubMed] [Google Scholar]
- Wolfe A. D., Cook T. M., Hahn F. E. Antibacterial nitroacridine, Nitroakridin 3582: effects on bacterial growth and macromolecular biosynthesis in vivo. J Bacteriol. 1971 Oct;108(1):320–327. doi: 10.1128/jb.108.1.320-327.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



