Abstract
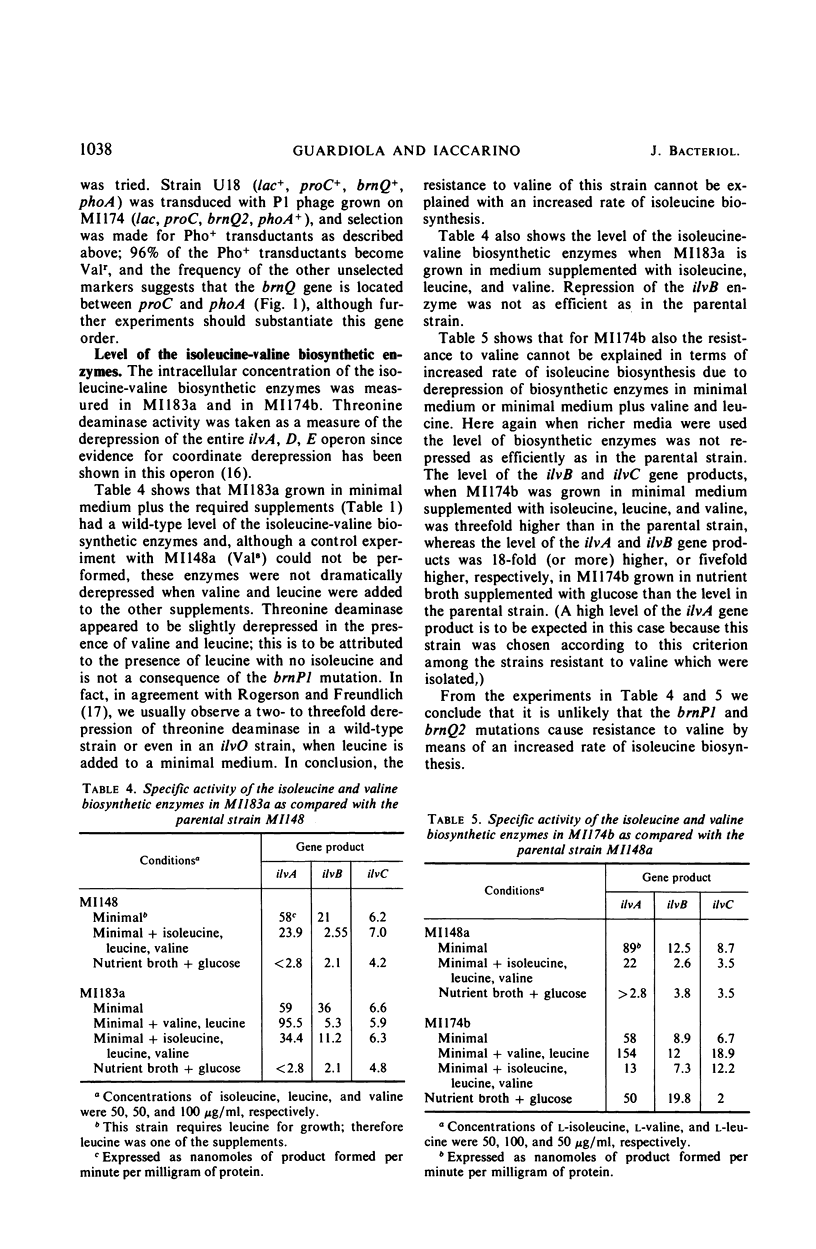
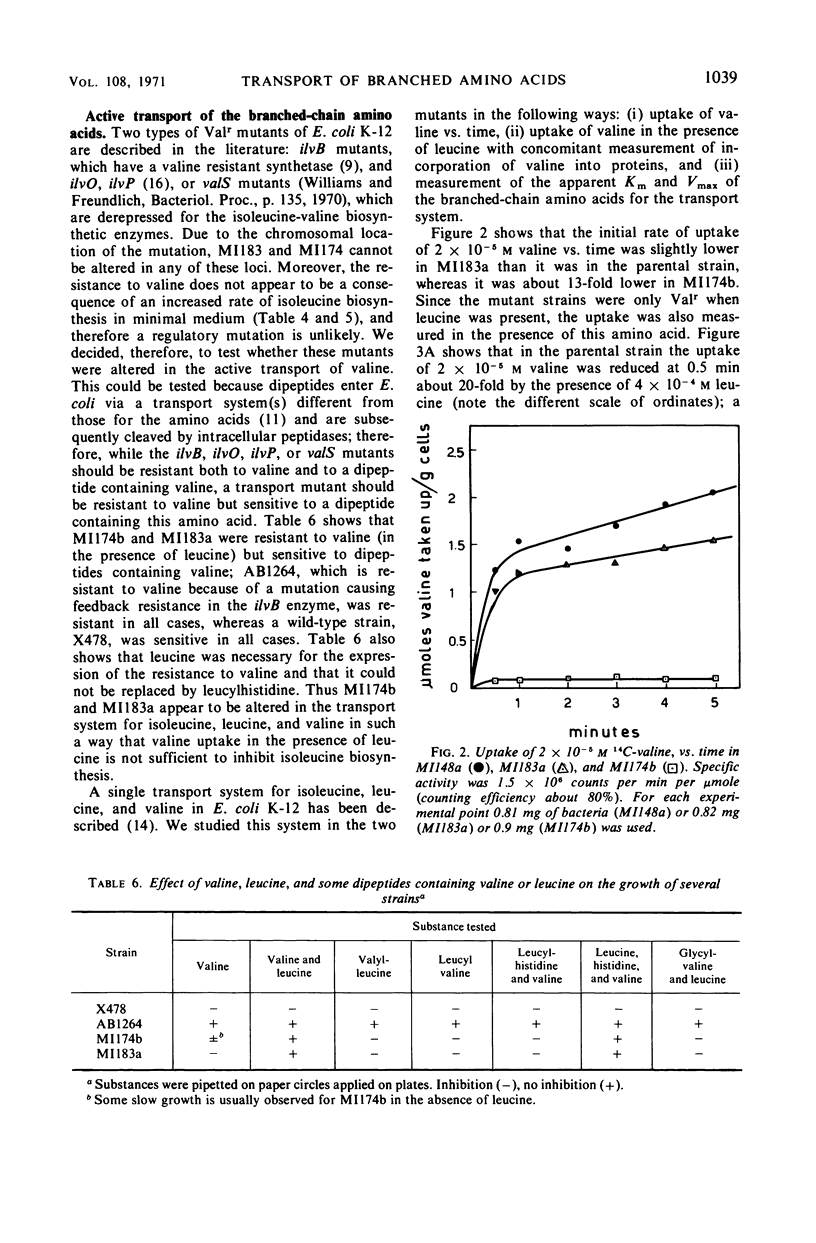
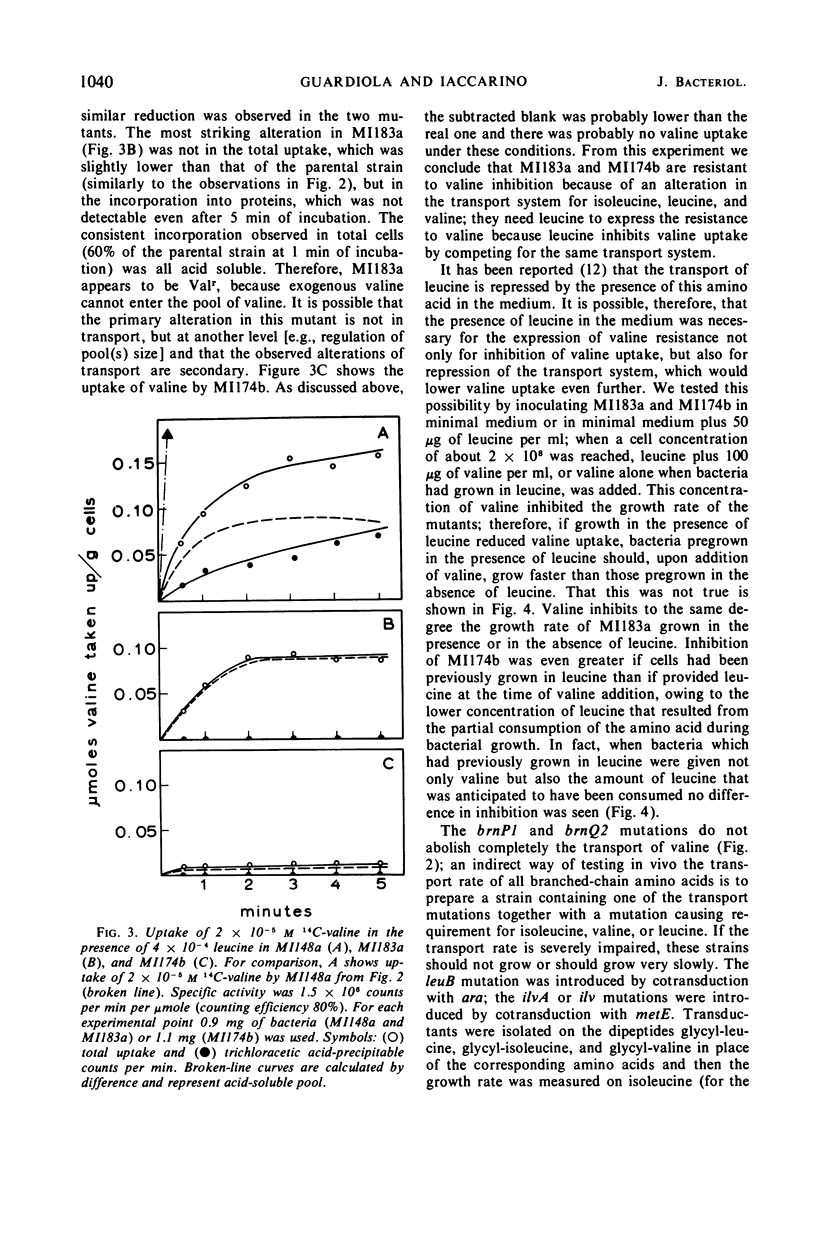
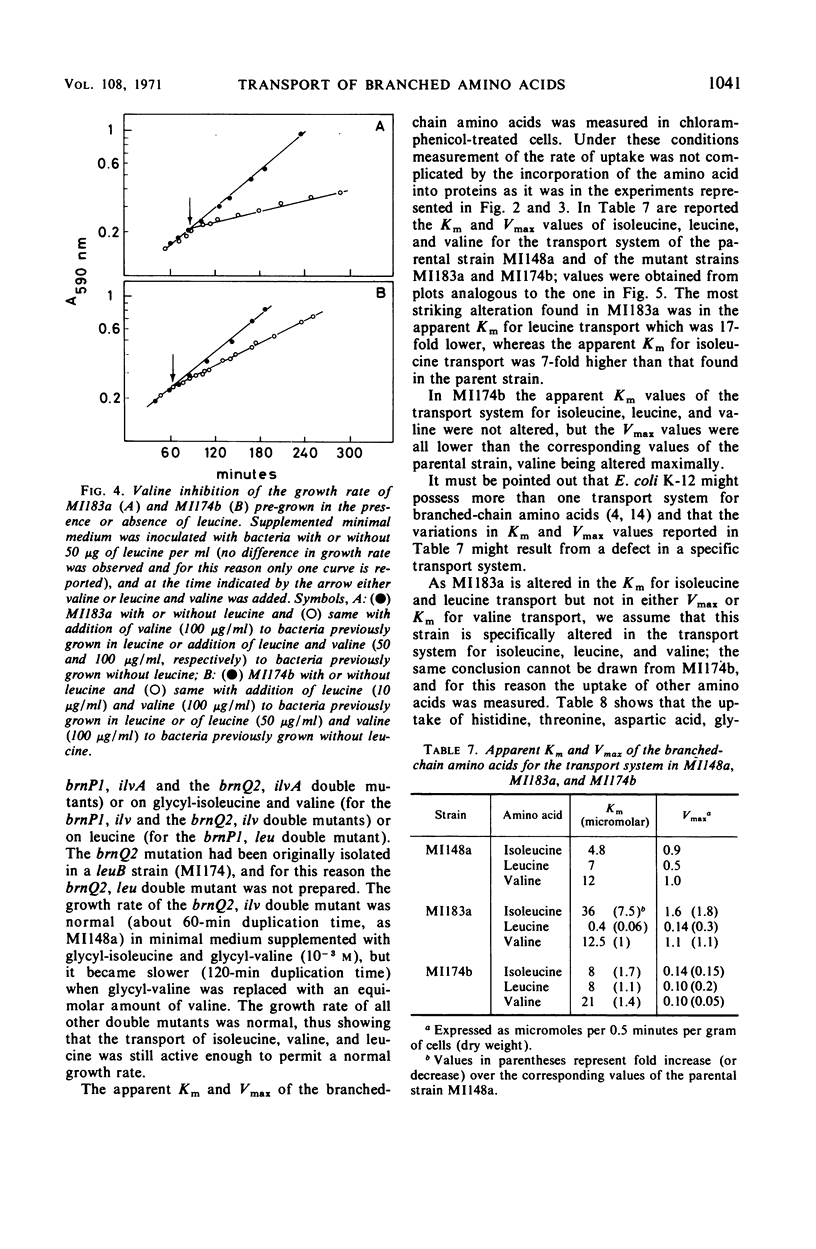
Two mutants of Escherichia coli K-12 are described which are resistant to the inhibition that valine exerts on the growth of E. coli. These mutants have lesions at two different loci on the chromosome. One of them, brnP, is linked to leu (87% cotransduction) and is located between leu and azi represented on the map at 1 min; the other, brnQ, is linked to phoA (96% cotransduction), probably between proC and phoA and represented at 10 min. These mutants are resistant to valine inhibition but are sensitive to dipeptides containing valine. Since it is known that dipeptides are taken up by E. coli through a transport system(s) different from those used by amino acids, this sensitivity to the peptides suggests an alteration in the active transport of valine. The mutants are resistant to valine only if leucine is present in the growth medium; the uptake of valine is less in both mutants than it is in wild-type E. coli, and it is reduced even further if leucine is present. Under these conditions the total uptake of valine is almost completely abolished in the brnQ mutant. The brnP mutant takes up about 60% as much valine as does the wild type, but no exogenous valine is incorporated into proteins. The apparent Km and Vmax of isoleucine, leucine, and valine for the transport system are reported; the brnP mutant, when compared to the wild type, has a sevenfold higher Km for isoleucine and a 17-fold lower Km for leucine; the Vmax for the three amino acids is reduced in the brnQ mutant, up to 20-fold for valine. The transport of arginine, aspartic acid, glycine, histidine, and threonine is not altered in the brnQ mutant under conditions in which that of the branched amino acids is. Evidence is reported that O-methyl-threonine enters E. coli through the transport system for branched amino acids, and that thiaisoleucine does not.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. Components of histidine transport: histidine-binding proteins and hisP protein. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1096–1103. doi: 10.1073/pnas.66.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Dwyer S. B., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XVI. Pattern of multivalent repression in strain K-12. J Bacteriol. 1968 May;95(5):1680–1684. doi: 10.1128/jb.95.5.1680-1684.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong C. E., Weiner J. H. Purification of a leucine-specific binding protein from Escherichia coli. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1076–1083. doi: 10.1016/0006-291x(70)90349-9. [DOI] [PubMed] [Google Scholar]
- Groves W. E., Davis F. C., Jr, Sells B. H. Spectrophotometric determination of microgram quantities of protein without nucleic acid interference. Anal Biochem. 1968 Feb;22(2):195–210. doi: 10.1016/0003-2697(68)90307-2. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Iaccarino M., Berg P. Isoleucine auxotrophy as a consequence of a mutationally altered isoleucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Feb;105(2):527–537. doi: 10.1128/jb.105.2.527-537.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAVITT R. I., UMBARGER H. E. Isoleucine and valine metabolism in Escherichia coli. XI. Valine inhibition of the growth of Escherichia coli strain K-12. J Bacteriol. 1962 Mar;83:624–630. doi: 10.1128/jb.83.3.624-630.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Penrose W. R., Nichoalds G. E., Piperno J. R., Oxender D. L. Purification and properties of a leucine-binding protein from Escherichia coli. J Biol Chem. 1968 Nov 25;243(22):5921–5928. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. I. GENETIC DEREPRESSION OF ENZYME FORMATION. J Bacteriol. 1964 Mar;87:566–573. doi: 10.1128/jb.87.3.566-573.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMAKRISHNAN T., ADELBERG E. A. REGULATORY MECHANISMS IN THE BIOSYNTHESIS OF ISOLEUCINE AND VALINE. II. IDENTIFICATION OF TWO OPERATOR GENES. J Bacteriol. 1965 Mar;89:654–660. doi: 10.1128/jb.89.3.654-660.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson A. C., Freundlich M. Control of isoleucine, valine and leucine biosynthesis. 8. Mechanism of growth inhibition by leucine in relaxed and stringent strains of Escherichia coli K-12. Biochim Biophys Acta. 1970 Apr 14;208(1):87–98. doi: 10.1016/0304-4165(70)90051-6. [DOI] [PubMed] [Google Scholar]
- Smulson M. E., Rabinovitz M., Breitman T. R. O-methylthreonine inhibition of growth and of threonine deaminase in Escherichia coli. J Bacteriol. 1967 Dec;94(6):1890–1895. doi: 10.1128/jb.94.6.1890-1895.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai A., Umbarger H. E. Isoleucine and valine metabolism of Escherichia coli. XIV. Effect of thiaisoleucine. J Bacteriol. 1968 May;95(5):1666–1671. doi: 10.1128/jb.95.5.1666-1671.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torriani A. Alkaline phosphatase subunits and their dimerization in vivo. J Bacteriol. 1968 Oct;96(4):1200–1207. doi: 10.1128/jb.96.4.1200-1207.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber G., Iaccarino M. Biochemical characterization of a mutant isoleucyl-transfer ribonucleic acid synthetase from Escherichia coli K-12. J Bacteriol. 1971 Sep;107(3):828–832. doi: 10.1128/jb.107.3.828-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Yagil E., Bracha M., Silberstein N. Further genetic mapping of the phoA-phoR region for alkaline phosphatase synthesis in Escherichia coli K 12. Mol Gen Genet. 1970;109(1):18–26. doi: 10.1007/BF00334043. [DOI] [PubMed] [Google Scholar]



