As memories age they become more resistant to disruption. This fact is the empirical basis of the concept of consolidation – the idea that the stability of the memory trace depends on processes that continue for some time after the initial induction of the trace. However, it is apparent to many contemporary researchers that unless the concept of consolidation is constrained in some way it has little value. Thus, today memory theorists differentiate between the concepts of cellular consolidation and systems consolidation.
Cellular consolidation is evoked when referring to the initial set of cellular/molecular processes that are engaged to support the strengthening of the synapses in a local circuit. They are assumed to operate on the scale of hours. Systems consolidation refers to processes that are involved in reducing the number of brain regions involved in the retrieval of a memory trace. This idea has evolved in the context of understanding the role of the hippocampus in memory (Marr, 1971; McClelland et al., 1995; Squire et al., 1984; Teyler and DeScenna, 1986). The basic idea is that some memories are established in a neural system composed of the hippocampus and neocortical regions that project to it. For some time after the memory is established its retrieval requires the participation of the hippocampus. However, eventually the memory trace can be retrieved without the participation of the hippocampus. During the intervening transition period the hippocampus, through its reciprocal interactions with these neocortical regions, facilitates consolidation of the memory trace in these regions
Systems consolidation is thought to operate on a much different time scale than cellular consolidation. The time scale on which systems consolidation operates, however, is largely unspecified and, it is disconcerting that it appears to depend on the data. In people with medial temporal lobe damage systems consolidation has been evoke to explain the sparing of memory traces at least thirty years old (see Nadel & Moscovitch, 1997 for a review). In rodents systems consolidation has been used to explain the sparing of memories several days to several months old prior to damage to the hippocampus. (Anagnostaras et al., 1999; Debiec et al., 2002; Kim and Fanselow, 1992; Maren et al., 1997; Clark et al., 2002; Kim et al., 1995; Ross and Eichenbaum, 2006; Winocur et al., 2001). Cellular consolidation is thought to operate over a period of hours.
The Morris Group’s Finding
In this context the recent report from the Morris group’s (Tse et al., 2007) laboratory deserves special attention because they claim support for the systems consolidation idea based on experiments in which the hippocampus was damaged either 3, 24 or 48 hours following the learning episode, a time frame usually associated with cellular consolidation. Damage to the hippocampus only produced amnesia when it occurred 3-hrs after the learning experience (see Figure 1). Thus, somewhere between 3 and 24 hours after training the hippocampus was no longer needed to retrieve the memory.
Figure 1.
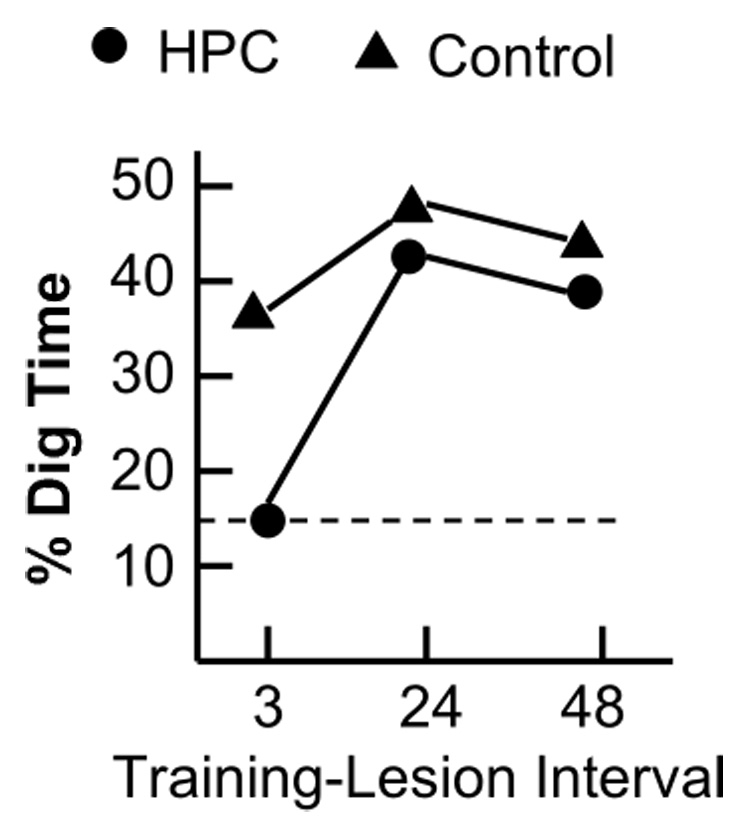
These data represent the main results from the Morris group’s experiment, summarized across the two key experiments (see Tse et al., 2007, Figure 2D and Figure 4). Damage to the hippocampus 3 hours after training produced amnesia for the newly learned paired associates but had no effect when it occurred 24 for or 48 hours later.
To quote the authors,
“The long sought upward gradient of remote spatial memory in rats when varying intervals of time are systematically scheduled before making hippocampal lesions is now definitely shown using a cued recall protocol for information acquired in one trial (Tse et al., 2007, p. 81)”.
In this claim the authors implicitly note that in recent years a number of laboratories, including theirs (Martin et al., 2005), have failed to find any evidence for systems consolidation using spatial learning tasks that depend on the hippocampus (Clark et al., 2005a,b; Sutherland et al., 2001). Moreover, if there is complete damage to the hippocampus, contextual fear memories are lost regardless of their age before the lesion (Lehmann, 2007). These data provide no support for the systems consolidation hypothesis. So there may be something special about their task that produces systems consolidation in just a few hours.
The Schema Experiment
A primary purpose of the Morris group’s experiments was to show that the availability of a “schema” facilitates the encoding and storage of memories for single events. Rats were given extensive training in a large arena that featured landmarks such as a stack of golf balls and a small pyramid. The floor of the area also was populated with a number of potential sand wells whose location could be defined in relation to the landmarks.
During training the rats learned several “paired associates” where the flavor of a food pellet specified the location of a sand well that contained a cache of same-flavor pellets. Specifically on a given trial the rat was placed into an entry box to the arena and given a distinctively flavored pellet. It was then released into the arena where 6 sand wells were uncovered. One of these wells contained 3 food pellets that matched the sample. There were 6 trials each session and on each trial a different flavored pellet was fed in the entry box and each pellet specified the location of the relevant sand well. Thus the rat was trained to use the flavor of the pellet to specify the location of a cache. Although there was nominally only one trial each session with each specific pair, according to Tse et al., (2007) the rats converted the single trial into 3 trials with each pair because when they located the food cache they would collect a single pellet then return to the entry box to consume it and then go back for the next pellet. Over the course of many training sessions the rats’ performance improved greatly and the number of wells visited prior to finding the correct one decreased from about 2.5 to a little over 1.
This initial long training phase established the schema. These rats then received a single trial with two new flavored pellets, each specifying a new sand well location. Twenty hours later the hippocampus of some rats was removed by injecting the excitotoxin ibotenic acid into multiple sites. After recovery these rats and control rats then received test trials with the old and new flavors. Remarkably their performance on both the old, well-trained pairs and the new single-trial trained pairs was as good as the no lesioned controls.
Thus, the extensive training with the old items established a schema or framework for the problem that allowed limited training to establish an enduring memory that could be retrieved without the hippocampus. A subsequent experiment, however, revealed that if the hippocampus was damaged only 3 hours after training only the memories of the old extensively trained paired associates survived.
A Cellular Consolidation Interpretation
The primary basis for the claim that these experiments support the systems consolidation hypothesis is that memories for the new minimally-trained paired associates survive hippocampal damage administered 24 hrs after training but do not survive when the hippocampus is damaged 3 hrs following training. Note that the consolidation time frame of these experiments (somewhere between 3 and 24 hrs the memory is consolidated) is one that is normally associated with cellular consolidation processes. One might suppose that the authors did not relate their findings to cellular consolidation because the other important result was that the age of the memory altered its dependency on the hippocampus for retrieval. Guided by a belief in systems consolidation theory (see Figure 2) the Morris group assumes that the results indicate that for a brief period (between 3 and 24 hours) retrieval of the most recently established memories required the hippocampus. The value of this interpretation, however, depends on how one interprets the effect of the lesion. A cellular consolidation interpretation would attribute the results to disruptive side effects of the lesion procedure itself (see Figure 3). This account assumes that the memories were established independently in the neocortex and that during the 3-to-24 hr period cellular consolidation processes were at work in the neocortical circuitry to stabilize the trace and thereby increase its resistance to disruption. Disruption of the cellular consolidation processes before the trace is stabilized would produce amnesia. Thus, this interpretation assumes that the temporally-graded amnesia provided by the Morris group’s experiments reflects the time required for cellular consolidation processes to produce a memory trace outside of the hippocampus that will not be disrupted by the set of abnormal neural events produced by massive damage to the hippocampus.
Figure 2.
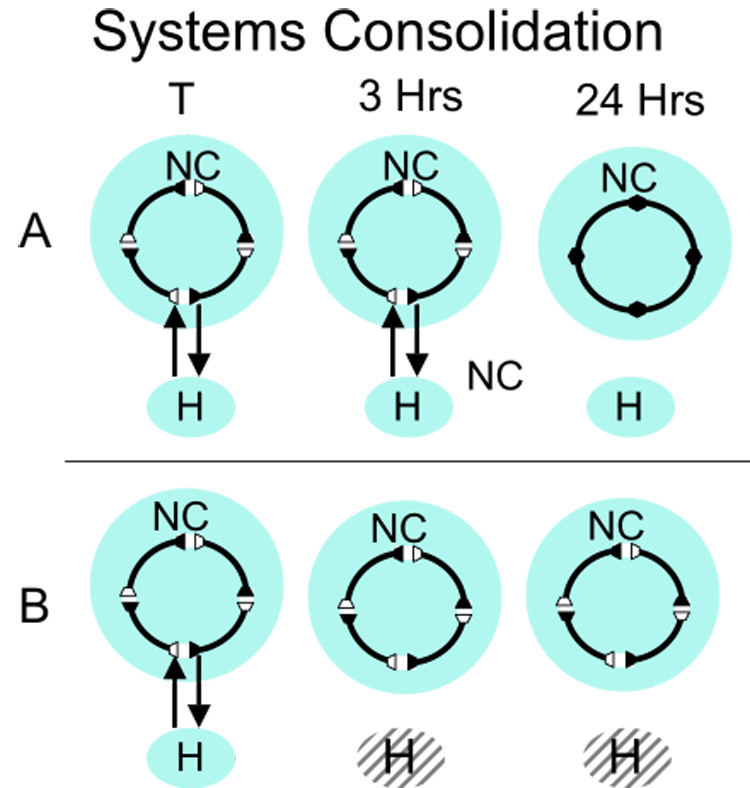
This figure illustrates the systems consolidation interpretation of the Morris group’s data. Training (T) initiates a memory trace that depends on both the neocortex and the hippocampus for retrieval. A. During a period between 3 and 24 hours interactions between the hippocampus strengthen the neocortical representation so that the memory at 24 hours no longer requires the hippocampus. B. Damage to the hippocampus 3 hours after training removes the hippocampus-neocortical interactions needed to consolidate the memory trace in the neocortex.
Figure 3.
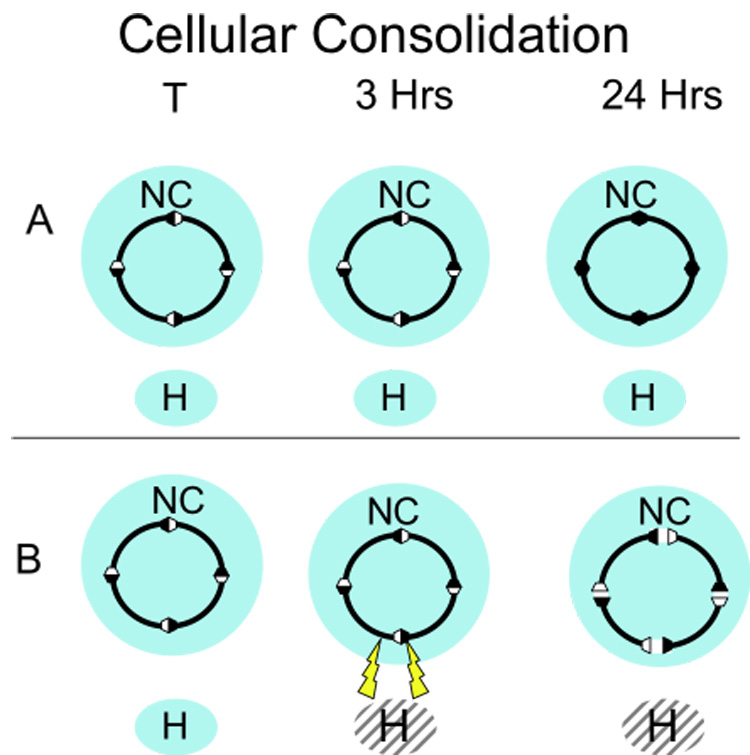
This figure illustrates a cellular consolidation interpretation of damage to the hippocampus. A. A trial initiates a memory trace in the neocortex. The stabilization of this trace depends on a cascade of cellular-molecular processes that are also initiated at that time. These processes are not dependent of the hippocampus and take between 3-to-24 hours to produce the stable trace. B. Massive damage to the hippocampus 3 hours after training produced by excitotoxic damage to the hippocampus sets of a set of abnormal neural processes that disrupts the normal cellular-molecular cascades in the neocortex that stabilizes the trace.
This hypothesis should not be lightly dismissed. Excitotoxic damage to the hippocampus does not simply remove the hippocampus. It sets off a cell-death cascade that is accompanied by synchronous excitatory discharge from the hippocampus into connected neocortex. The resultant effects would likely be: (a) excessive release of transmitters (see Jacobson et al., 1997) into terminal fields of hippocampal outputs, (b) altered patterns of immediate early gene expression (Glenn et al. 2005) and (c) altered postsynaptic intracellular signals involved in stabilizing the synaptic underpinnings of the memory trace (see Anagnostaros et al., 2001, p. 11, for a similar view).
The Role of the Schema
Once the paired associate memory was stabilized, it did not require the hippocampus for retrieval. Thus the memory clearly resided outside of the hippocampus. Extensive training, however, was necessary to obtain these results. This training may well have established a schema for rapidly storing the paired-associate memory in the neocortex. The systems consolidation account assumes that this now hippocampus-independent memory trace was not immediately available and initially was retrieved with the aid of the hippocampus. The cellular consolidation interpretation, in contrast, implies that the retrieval of this memory from extensively trained rats with intact hippocampi was never dependent on the hippocampus.
It is noteworthy that rats with hippocampus damage could not learn new sets of paired associates when they were trained in a novel room with new landmarks in the arena (Tse et al., 2007). Thus, the hippocampus is necessary to produce a schema for solving the paired-associates task. However, this fact does not necessarily mean that the retrieval of recently acquired memories by well-trained intact rats temporarily depended on the hippocampus. In fact there is some evidence provided in the supplementary material (see Tse et al., 2007, Figure S6A) that for a few sessions following damage to the hippocampus, when they were trained on new pairs in the old arena, the rats performed above chance. This result is consistent with the cellular consolidation interpretation.
Exactly why damage to the hippocampus interferes with rats learning a new flavor map is not clear. The systems consolidation account assumes that the hippocampus is necessary for storing the map in the neocortex. However, the poor acquisition of the new map might be due to how the lesion altered the rat’s sampling behavior. For example, Whishaw has reported that hippocampal damage is associated with abnormal pellet retrieval behavior in an open field (e.g. Whishaw, 1993; Whishaw & Tomie, 1997). A disorganization of pellet retrieval may interfere with the initial acquisition of neocortical schemas.
Conclusion
In summary, the Morris group has provided a fascinating set of data that suggest a beneficial role for schema in the memory encoding process. However, in our view, only by passing over the abnormal neural consequences that widespread excitotoxic damage to the hippocampus can produce can one conclude that the temporally graded amnesia they report supports a systems consolidation view. The time frame (somewhere between 3 and 24 hours) in which consolidation occurred was well within the period normally ascribed to cellular consolidation. Moreover, it is reasonable to suppose that damage to the hippocampus would disrupt these cellular consolidation processes. For these reasons we propose that the temporal gradient observed by the Morris group was determined by the time required for cellular consolidation processes operating in the neocortical circuits to stabilize the memory trace and not the time required for systems consolidation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jerry W. Rudy, University of Colorado
Robert J. Sutherland, University of Lethbridge
References
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. Journal of Neuroscience. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11(1):8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Zola SM, Squire LR. Anterograde amnesia and temporally graded retrograde amnesia for a nonspatial memory task after lesions of hippocampus and subiculum. Journal of Neuroscience. 2002;22:4663–4669. doi: 10.1523/JNEUROSCI.22-11-04663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Hippocampus and remote spatial memory in rats. Hippocampus. 2005a;15:260–272. doi: 10.1002/hipo.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Broadbent NJ, Squire LR. Impaired remote spatial memory after hippocampal lesions despite extensive training beginning early in life. Hippocampus. 2005b;15:340–346. doi: 10.1002/hipo.20076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Lehmann H, Mumby DG, Woodside B. Differential fos expression following aspiration, electrolytic, or excitotoxic lesions of the perirhinal cortex in rats. Behavioral Neuroscience. 2005;119:806–813. doi: 10.1037/0735-7044.119.3.806. [DOI] [PubMed] [Google Scholar]
- Jacobsson SO, Cassel GE, Karlsson BM, Sellstrom A, Persson SA. Release of dopamine, GABA and EAA in rats during intrastriatal perfusion with kainic acid, NMDA and soman: a comparative microdialysis study. Archives of Toxicology. 1997;71:756–765. doi: 10.1007/s002040050458. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Lacanilao S, Sutherland RJ. Complete or partial hippocampal damage produces equivalent retrograde amnesia for remote contextual fear memories. European Journal of Neuroscience. 2007;5:1278–1286. doi: 10.1111/j.1460-9568.2007.05374.x. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioral Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philosopichal Transactions of the Royal Society of London, Series B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- Martin SJ, de Hoz L, Morris RG. Retrograde amnesia: neither partial nor complete hippocampal lesions in rats result in preferential sparing of remote spatial memory, even after reminding. Neuropsychology. 2005;43:609–624. doi: 10.1016/j.neuropsychologia.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion Neurobiology. 1997;2:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Ross RS, Eichenbaum H. Dynamics of hippocampal and cortical activation during consolidation of a nonspatial memory. Journal of Neuroscience. 2006:264852–264859. doi: 10.1523/JNEUROSCI.0659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Cohen NJ, Nadel L. The medial temporal region and memory consolidation: a new hypothesis. In: Weingartner H, Parker E, editors. Memory consolidation. Hillsdale, New Jersey: Erlbaum; 1984. pp. 185–210. [Google Scholar]
- Sutherland RJ, Weisend MP, Mumby D, Astur RS, Hanlon FM, Koerner A, Thomas MJ, Wu Y, Moses SN, Cole C. Retrograde amnesia after hippocampal damage: recent vs. remote memories in two tasks. Hippocampus. 2001;11:27–42. doi: 10.1002/1098-1063(2001)11:1<27::AID-HIPO1017>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, Bethus I, Spooner PA, Wood ER, Witter MP, Morris RG. Schemas and memory consolidation. Science. 2007;316:76–82. doi: 10.1126/science.1135935. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behavioral Neuroscience. 1986;100:147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Activation, travel distance, and environmental change influence food carrying in rats with hippocampal, medial thalamic, and septal lesions. Implications for studeis on hoarding and theories of hippocampal function. Hippocampus. 1993;3:373–385. doi: 10.1002/hipo.450030311. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA. Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat food carrying task. Behav. Brain Res. 1997;89(1–2):87–97. doi: 10.1016/s0166-4328(97)00068-5. [DOI] [PubMed] [Google Scholar]
- Winocur G, McDonald RM, Moscovitich M. Anterograde and retrograde amnesia in rats with large hippocampal lesions. Hippocampus. 2001;11:18–26. doi: 10.1002/1098-1063(2001)11:1<18::AID-HIPO1016>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]


