Abstract
The organic solute and steroid transporter (OST/Ost) is a unique membrane transport protein heterodimer composed of subunits designated alpha and beta, that transports conjugated steroids and prostaglandin E2 across the plasma membrane. Ost was first identified in the liver of the cartilaginous fish Leucoraja erinacea, the little skate, and subsequently was found in many other species, including humans and rodents. The present study describes the isolation of a new cell line, LEE-1, derived from an early embryo of L. erinacea, and characterizes the expression of Ost in these cells. The mRNA size and amino acid sequence of Ost-beta in LEE-1 was identical to that previously reported for Ost-beta from skate liver, and the primary structure was identical to that of the spiny dogfish shark (Squalus acanthias) with the exception of a single amino acid. Ost-beta was found both on the plasma membrane and intracellularly in LEE-1 cells, consistent with its localization in other cell types. Interestingly, arachidonic acid, the precursor to eiconsanoids, strongly induced Ost-beta expression in LEE-1 cells and a lipid mixture containing arachidonic acid also induced Ost-alpha. Overall, the present study describes the isolation of a novel marine cell line, and shows that this cell line expresses relatively high levels of Ost when cultured in the presence of arachidonic acid. Although the function of this transport protein in embryo-derived cells is unknown, it may play a role in the disposition of eicosanoids or steroid-derived molecules.
Keywords: Ost, organic solute and steroid transporter, little skate, L. erinacea, LEE-1 cell line, arachidonic acid
1. Introduction
The organic solute and steroid transporter (Ost) is a heterodimeric transmembrane anion transporter originally identified as a bile salt transporter in the liver of the little skate, Leucoraja erinacea (Wang et al., 2001; Seward et al., 2003). The complex is composed of two subunits, Ost-alpha and Ost-beta. The larger subunit, Ost-alpha, is a predicted seven-transmembrane protein, whereas Ost-beta has a single transmembrane domain, with structural relationship to G-protein coupled receptors and receptor activity-modifying proteins (RAMPs) (Ballatori, 2005; Parameswaran and Spielman, 2006). When expressed in Xenopus oocytes, the Ost complex mediates transport of conjugated steroids and eicosanoids (Wang et al., 2001), suggesting that it may play a role in transport of both cellular metabolites and signaling molecules (e.g., steroids and prostaglandins) (Ballatori 2005). In mammals, Ost is most abundant in the liver, kidney and intestine, and this transporter is thought to mediate ileal basolateral reabsorption of bile acids and conjugated steroids (Ballatori et al., 2005; Dawson et al., 2005; Li et al., 2007). Both Ost subunits are induced by the activated farnesoid X receptor (FXR), which modulates activity of other proteins related to bile acid homeostasis (Boyer et al., 2006; Landrier et al., 2006 Lee et al., 2006 Frankenberg et al., 2006; Zollner et al., 2006).
Detailed information is beginning to accumulate on Ost expression and regulation in adult mammals, but little is known about the expression or function of this transporter in comparative evolutionary or developmental biology of other families of vertebrates. The present study describes the isolation of a novel multipassage, continuously proliferating cell line (LEE-1) derived from an early embryo of the little skate, and examines expression and regulation of Ost-alpha/beta in these cells in vitro, as well as comparing amino acid sequence information among nonmammalian vertebrates. The amino acid sequence and mRNA size of Ost-beta in these cells was identical to that found in skate liver, and the primary structure was 99% identical to that of the dogfish shark, Squalus acanthias. Expression of Ost-beta was detected by specific antibody, and induction was observed in the presence of fatty acids, most notably arachidonic acid, the precursor of eicosanoids such as prostaglandin E2, an Ost substrate (Wang et al., 2001).
2. Materials and Methods
2.1. Isolation and culture of the LEE-1 skate embryo cell line
The basal nutrient medium used for culture was LDF (Helmrich and Barnes, 1999; Barnes et al., 2007), a mixture of 50% Dulbecco-modified medium (GIBCO), 35% L-15 (Sigma), and 15% Ham's F-12 (GIBCO), containing sodium bicarbonate (0.18 mg/mL) and 15 mM HEPES buffer (pH 7.2). Cultures were initiated from a stage 28 skate embryo collected at the Mount Desert Island Biological Laboratory, Salsbury Cove, Maine. At this stage neither eye pigmentation nor external gill filaments have developed (Ballard et al., 1993). Kidney and heart development have been initiated, but liver development has not begun. The embryo was removed from the egg casing and yolk, and washed in LDF medium containing a high-concentration antibiotic solution (1250 units penicillin G, 1.25 g streptomycin sulfate, 1.25 g neomycin sulfate, 12,500 units bacitracin). Tissue was disaggregated for 30–45 min in LDF medium containing 0.01 mg/mL dispase II (Roche, Indianapolis, IN, USA), minced, and washed again in LDF medium. For primary culture, single cells, small groups of cells and larger tissue clumps were dispensed into four collagen-coated wells of a 24-well plate (2.5 mg/mL collagen; Cohesion, USA). The antibiotic concentrations at the time of primary culture were penicillin G (200 units/mL), streptomycin sulfate (200 µg/mL), and ampicillin (25 µg/mL), and were maintained at this level throughout further culture. Cells were maintained in ambient carbon dioxide levels at 18°C.
Additional supplements added to the basal nutrient medium were insulin (10 µg/mL, Sigma), transferrin (10 µg/mL, Sigma), selenous acid (10 nM, Sigma), human recombinant epidermal growth factor (EGF, 50 ng/mL, R&D), basic fibroblast growth factor (FGF, 50 ng/mL, R&D), L-glutamine (0.2 mM, Gibco), chemically defined lipids (CDL, 1:1000, Gibco), sea water (4%), non-essential and essential amino acids (1:1000, Gibco), and heat-inactivated fetal bovine serum (FBS, 2%, Hyclone, Logan, UT, USA). Subsequently, cells were passaged using collagenase (2 mg/mL, Sigma) in trypsin/EDTA (0.2%/1 mM, Sigma). Maximum growth rate was approximately 10 days per population doubling. This slow growth rate is consistent with the incubation temperature and cold water preference of the organism. Cell culture plates and flasks were obtained from Nunc (Rochester, NY, USA), Corning (Corning, NY, USA), or Becton Dickinson (Franklin Lakes, NJ, USA).
2.2. RNA isolation
LEE-1 cells in 24 cm2 flasks (2 × 106 cells/flask) were pooled by scraping into Trizol (Invitrogen, USA), and total RNA was isolated according to the manufacturer’s instructions. RNA from skate tissues was prepared by mincing and homogenizing in Trizol. Total RNA was incubated with DNase I to remove contaminant genomic DNA traces, and reverse-transcribed using an oligo(dT)20 primer and the SuperScript III first-strand synthesis system (Invitrogen) according to the manufacturer’s instructions. Primers used for PCR amplification are shown in Table 1.
Table 1.
PCR primer sequences
| Ost Beta primers (5’→3’) |
| 1: Forward: TGGAAGATCCCACTAACTGG |
| 1: Reverse: AGCATCTGGTCTGTTTCAGC |
| Amplification Product Size 226 bp |
| 2: Forward: TTGCTTCATCCTGTGTCTGC |
| 2: Reverse: GCTGTCGGTGTAAAGGAAGC |
| Amplification Product Size 502 bp |
| 3: Forward: CTCCAACTGGAAAACCAGACATGAG |
| 3: Reverse: CTGTAGCTGGTTGCTACACATCATC |
| Amplification Product Size 581 bp |
| Ost Alpha primer (5’→3’) |
| 1: Forward: GTTTCTGAGGCGTCGTTC |
| 1: Reverse: TGGGTCATAGAGGCCGTT |
| Amplification Product Size 542 bp |
| Elongation factor 1-alpha primer (5’→3’) |
| 1: Forward TGAACGTGAGCGTGGAATTA (amplicon size 249 bp). |
| 1: Reverse:TAGGCAAGAAGGGCATGTTC |
| Amplification Product Size: 228 bp |
Skate Ost-specific and EF-1 alpha elongation factor primers were designed using Primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi).
2.3. Polymerase chain reaction
Amplification was performed with 0.1 uM of each primer. Reverse transcription (RT)-PCR was carried out with the Advantage GC2 PCR kit (BD Biosciences, USA). Conditions were: initial denaturation for one cycle of 94 °C for 1 min 45 s, 35 PCR cycles (denaturating, 94 °C, 30 s, annealing, 60 °C, 30 s, extension, 72 °C, 1 min) and final extension for one cycle at 72 °C for 10 min. Amplified fragments showing the expected size were excised from ethidium bromide-stained agarose gels (1.5 %) and purified with a GenElute spin-column (Sigma). The fragments were subcloned into pGEM-T Easy vector (Promega, USA) according to the manufacturer’s instructions and .transformed into competent Escherichia coli cells, JM109. Plasmid DNA sequences were determined in both directions and results analyzed using the Lasergene (DNAstar, USA) DNA and protein sequence analysis program to confirm that the amino acid sequence of the amplification products were of the skate sequence predicted.
2.4. Northern blot
Antisense-digoxigenin (DIG) probe was synthesized by using a DIG northern starter kit from Roche (Switzerland). The antisense probe for Ost beta was generated by in vitro transcription from a linearized plasmid containing an Ost insert generated by primers 3F/3R (Table 1). This probe represents the entire coding sequence for Ost-beta. Total RNA (3 µg) was denatured at 65 °C for 10 min in RNA sample loading buffer, applied to a 1% agarose gel containing 2% formaldehyde and MESA buffer (Sigma), and transferred to a positively charged nylon membrane (Roche, Switzerland). The RNA was fixed to the membrane by baking at 80 °C for 2 h. The membrane was prehybridized at 68 °C for 3 h in DIG Easy Hyb (Roche) and hybridized at 68 °C overnight with the DIG-labeled Ost-beta. After hybridization, the membrane was washed three times for 10 min in 2x SSC/0.1% SDS at room temperature, and three times for 30 min in 0.1x SSC/0.1% SDS at 68 °C. The hybridization signal was detected and visualized by chemiluminescence using a DIG wash and blocking buffer set, anti-Digoxigenin-AP and CDP-star (Roche, Switzerland), and X-ray film (Kodak, Japan). The exposure time was 5 min.
A peptide corresponding to amino acids 34–47 of skate Ost-beta, ETIDIEKQNMTGER(C), was synthesized, coupled via added C-terminal cysteine residues to keyhole limpet hemocyanin using sulfosuccinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate, and used to immunize New Zealand White rabbits (AnaSpec, Inc). LEE-1 cells were transferred to 4-well chamber slides (8 × 104 cells/well) (Nunc, USA) and incubated for 7 days at 18°C. The slide was washed 5 times with LDF medium, fixed with ice cold methanol for 5 min and washed twice with PBS. Cells were incubated with a 1:500 dilution of immune and pre-immune serum with agitation for 1 hour at room temperature and the slide was washed 5 times with PBS. Goat anti-rabbit Texas Red-conjugated second antibody (Molecular Probes, USA) was added for one hour at room temperature at a dilution of 1:200 in blocking solution (PBS, 1% BSA, 0.1% Triton X-100). Slides were washed 5 times with PBS, mounted with Fluoromount-G (Southern Biotech, USA) and fluorescence examined with a Zeiss Axiovert microscope.
2.5. Quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from each sample and cDNA synthesized. The expression of LEE-1 Ost was compared by qPCR with skate elongation factor EF-1 alpha as the normalizer. The qPCR assay was performed in a 200-µL-tube with a total volume of 25-µL. The reaction mixture contained 12.5 µL of 2X master mix of Brilliant® SYBR® Green QPCR Reagent (STRATAGENE Co., Inc., La Jolla, CA), 0.375 µl of 1:500 diluted ROX reference dye (Stratagene), 100 nM of 5’and 3’ primer, and 0.5 µL of cDNA. Amplification and detection was carried out with a Mx 3000P® QPCR system (Stratagene). Conditions were: 95°C for 10 min, 40 cycles of template DNA denaturation at 95 °C for 15 s, primer-template annealing at 55 °C for 20 s, and primer extension at 72 °C for 20 s. Data were analyzed with MX pro software (Stratagene).
2.6. In situ hybridization
The probes for Ost alpha were generated by in vitro transcription from a linearized plasmid containing an Ost insert generated by primers shown in Table 1. Amplified OST-alpha PCR products were subcloned, and the plasmid DNA was linearized and purified by using the Wizard SV Gel and PCR Clean-Up System (Promega, USA). Antisense and sense probes were labeled with digoxigenin SP6 or T7 RNA polymerases (Roche, Switzerland). The digoxigenin-labeled probe was detected by anti-digoxigenin -AP and a 4-nitro blue tetrazolium chloride, 5-bromo-4-chloro-3'-indolyphosphate-p-toluidine mixture. The concentration of labeled probes was determined by dot blot analysis, and identical amounts of each probe were used for hybridization. Fixation of LEE-1 cells was accomplished by washing once with culture medium and incubation for 10 min at room temperature in 4% paraformaldehyde and 10% acetic acid in PBS. Cells were washed twice with PBS and permeabilized with 70% ethanol overnight at 4 °C.
The following day, cells were rehydrated for 5 minutes at room temperature in 2XSSC (pH 8.0), 50% formamide, and prehybridized for one h at 37 °C in hybridization solution (50% formamide, 1.3× SSC, pH 4.5, 5 mM EDTA, pH 8.0, 50 mg/mL tRNA, 0.2% Tween-20, 0.5% CHAPS and 100 mg/mL heparin). Hybridization was performed overnight at 37 °C. Cells were washed 3 times for 30 min at 50 °C in wash solution, 50% formamide, 1.3× SSC, pH 4.5, 0.2% Tween-20, and then washed 3 times for 30 min each time at 50 °C in 50% formamide, 1XSSC, pH 4.5 and 0.2% Tween-20. Final wash was three times for 5 min each at room temperature in 137 mM NaCl, 2.7 mM KCl and 0.1% Tween-20 in 25 mM Tris-HCl, pH 7.5 (TBST). Cells were blocked for one hour at room temperature in TBST with 10% heat-inactivated sheep serum and finally incubated at 4 °C overnight in TBST with 1% sheep serum and anti-Digoxigenin-AP (Roche, Switzerland). Excess antibody was removed by a series of washes in TBST. Following these washes, cells were washed 3 times at room temperature in 100 mM NaCl, 100 mM Tris-HCl, pH 9.5, 50 mM MgCl2 and 0.1% Tween-20) and then incubated with a mixture of 4-nitro blue tetrazolium chloride and 5-bromo-4-chloro-3'-indolyphosphate-p-toluidine (Roche, Switzerland). After the color reaction had reached optimal staining, the reaction was stopped by washing the cells 2 times for 10 min at room temperature in the above solution, followed by two 10 min washes at room temperature in PBS, with 0.1% Tween-20).
3. Results
3.1. The LEE-1 Leucoraja erinacea embryo-derived cell line
The medium developed for the LEE-1 cell line represents a complicated formulation of nutrients, growth factors and other growth-promoting molecules. Cells for the LEE-1 cell line were derived from an early-stage skate embryo at a developmental point at which gill filaments and eye pigmentation were not yet observed. Based on the developmental scheme of Ballard et al., (1993), the embryo was approximately at stage 28. This is prior to hepatogenesis, although renal and cardiac organogenesis has initiated. The Ballard staging is based on that of the spotted dogfish shark. It is the only source of staging information for elasmobranches (sharks, skates and rays), and probably is of general utility.
Cells were grown in a basal nutrient medium modified for elasmobranch cell culture (Parton et al., 2007; Barnes et al., 2007). This basal formulation was further supplemented with insulin, transferrin, EGF, FGF, FBS and a commercially available mixture of chemically defined lipids (CDL) as described in Materials and Methods. Among these supplements, the most important for plating and growth were FGF, EGF and CDL. Cells were passaged by enzymatic digestion of cultures to produce single cell suspensions prior to replating. No evidence for tight junctions that would result in cells maintaining multicellular aggregrates in suspension or culture was observed. Maximum cell density was approximately 2 × 105/cm2. The cells have been cultured for two years in a continuously proliferative state and can be cryogenically preserved. The population has not been cloned, but evidence indicates that, at least for expression of Ost-beta, the cells behave in a homogeneous manner. Photomicrographs of the cells are shown in several of the figures below.
3.2. Expression of Ost in the LEE-1 cell line
Because Ost was first identified by using a comparative approach in the identification of xenobiotic and anionic membrane transport proteins in the liver of the little skate (Wang et al., 2001), we examined the LEE-1 cell line to determine if it might serve as a model for expression and studies of regulation and function of Ost in vitro. Using primers derived from previously published work on xenobiotic/anion membrane transport proteins in skate liver cells, Ost-beta mRNA expression was detected in these cells by RT-PCR (Fig.1). Other transporters previously identified in skate liver: the bile salt export pump, multidrug resistance-associated protein-2 and organic anion transport protein (Cai et al. 2001; 2002; 2003), were not detected (not shown). Sequencing of the PCR products amplified by the use of primers specific for Ost-beta confirmed the identity as representative of mRNA for this protein (Fig. 1). The amplification product generated from primer set 3 (Table 1) included the entire coding region for Ost-beta, and this sequence was identical to that reported by Wang et al., (2001).
Figure 1.
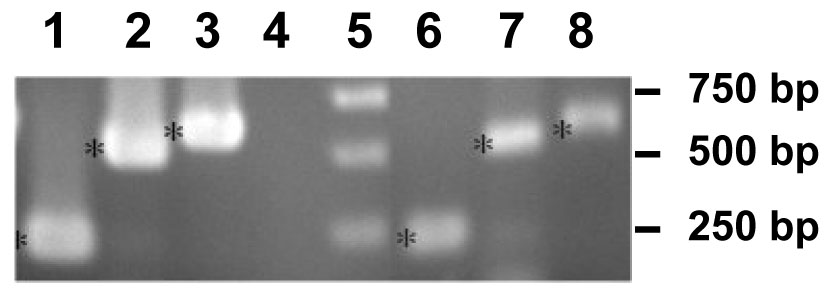
Reverse-transcription PCR of mRNA from skate liver and the LEE-1 skate embryo cell line. Primer sequences are listed in Table 1. (1), skate liver primers, 1-Forward/1-Reverse primers; (2), skate liver, primers 2-Forward/2-Reverse; (3), skate liver, primers 3-Forward/3-Reverse; (4) control (no sample) (5), molecular weight markers; (6), LEE-1 cell line, primers 1-Forward/1-Reverse; (7), LEE-1 cell line, primers 2-Forward/2-Reverse; (8), LEE-1 cell line, primers 3-Forward/3-Reverse. PCR was carried out as described in Materials and Methods.
Northern blot of RNA for OST beta from the LEE-1 cell line showed a single band at approximately 0.9 kb, equivalent to that found for this message identified directly from skate liver (Fig. 2) (Wang et al., 2001). Quantitative PCR of a range of skate tissues found Ost beta to be present in tissues of adult animals, as well as the Lee-1 cells and embryonic skate (Fig. 3). RT-PCR of skate tissues showed the presence of OST alpha in liver, spiral valve. gill and heart, as well as in the LEE-1 cell line. Expression of Ost-beta in LEE-1 cells at the protein level was confirmed by immunohistochemistry (Fig. 4). Attempts to compare protein levels between LEE-1 cells and skate liver by Western blot were unsuccessful because of unsuitability of the antibody in this assay. Antibody to OST alpha is not available. Although Ost-beta contains a transmembrane domain that anchors it to the basolateral membrane of polarized cells (Wang et al., 2001; Ballatori et al., 2007; Sun et al., 2007), immunoreactivity for Ost-beta was also detected intracellularly, especially in the region of the endoplasmic reticulum, a phenomenon that has been observed previously in mammalian cells (Dawson et al., 2005; Li et al., 2007; Sun et al., 2007). No evidence exists to this point that LEE-1 cells are in a membrane-polarized state under the culture conditions used.
Figure 2.
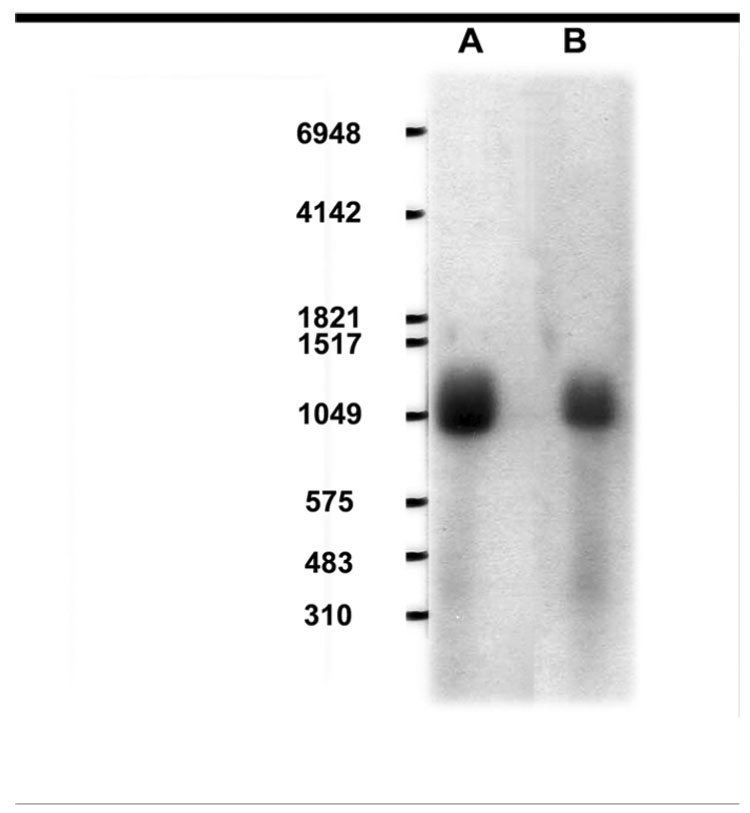
Northern blot of Ost-beta mRNA in skate liver and LEE-1 cell line. (A), LEE-1 cell line; (B), skate liver. Procedures were as described in Materials and Methods.
Figure 3.
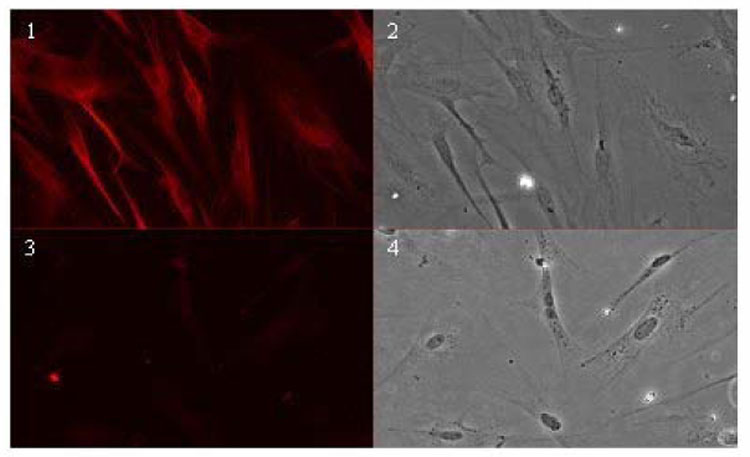
Immunohistochemical detection of Ost-beta in LEE-1 cells. (1), cells treated with anti-skate Ost beta antiserum ; (2), phase photomicrograph of the same field; (3), cells treated with preimmune serum ; (4), phase photomicrograph of the same field. Immunolocalization of Ost-beta was carried out as described in Materials and Methods.
Figure 4.
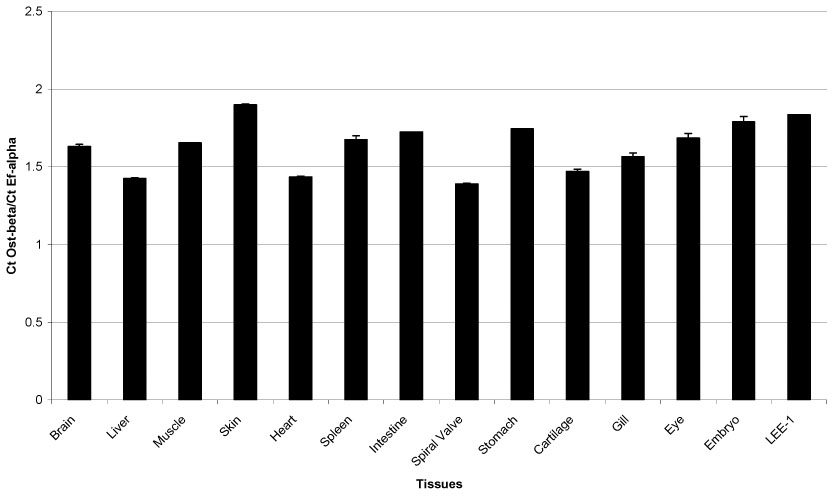
Quantitative PCR analysis of Ost-beta in skate tissue. Primer set used was F1/R1 as shown in Table 1. Procedures are given in Materials and Methods.
3.3. Sequence comparisons
Using a similar approach, the Ost-beta expressed by the SAE shark (S. acanthias) embryo-derived cell line was also sequenced. This recently derived line is the first continuously proliferating cell line from a cartilaginous fish (Parton et al., 2007; Forest et al., 2007). S. acanthias and L. erinacea diverged approximately 200 million years ago. However, Ost-beta from the two species was nearly identical, with only a single amino acid difference in the primary sequence (Fig. 5). The shark Ost-beta sequence was confirmed by analysis of an Ost-beta expressed-sequence tag derived from the shark rectal gland. This sequence recently was contributed to Genbank (accession no. EF059803).
Figure 5.
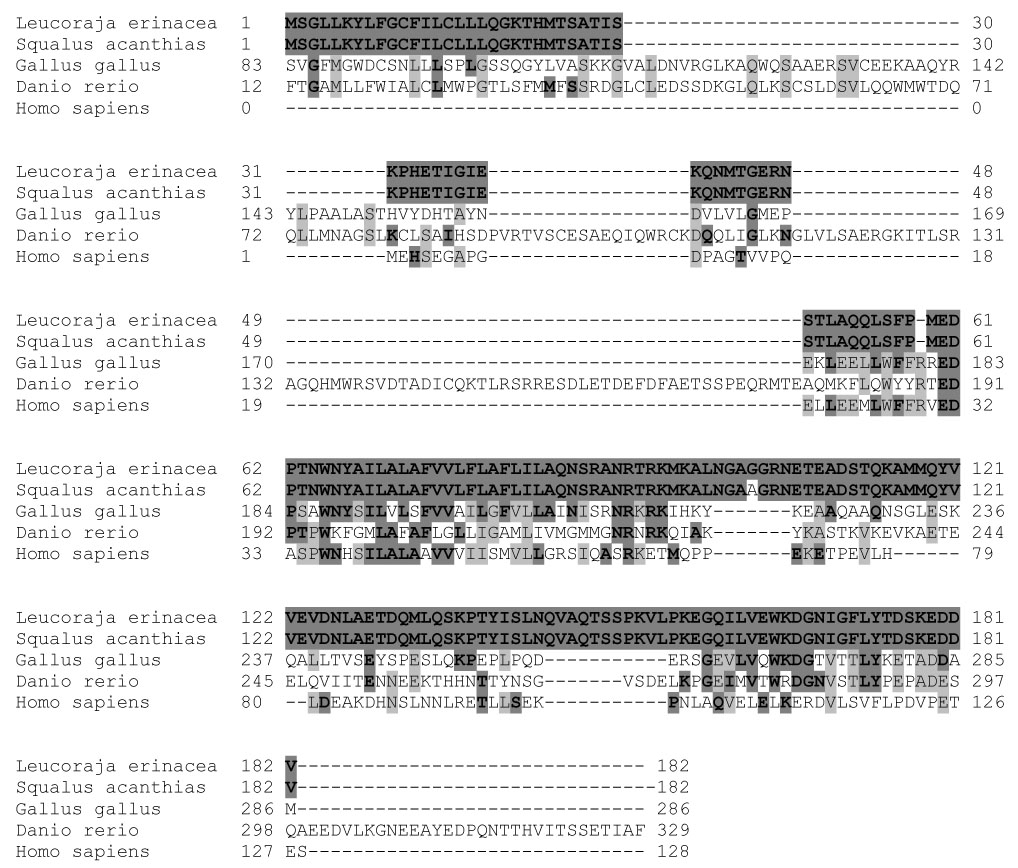
Sequence alignment of primary structure of Ost-beta. Bold shadings indicate amino acid identity to the skate sequence; gray shadings indicate identity among amino acids of the other species.
The shark and skate sequences were compared to Ost-beta sequences from several other species: human (Ballatori 2005), chicken (Gallus gallus) and zebrafish (Danio rerio). Sequence comparisons extended the observation previously reported with skate, mouse, rat and human, that conservation exists around the predicted transmembrane portion of the protein. An area of amino acid conservation was also observed at the carboxy- terminal portion of the protein. The Danio sequence was striking in the degree of additional sequence similarity in the amino terminal portion of the protein prior to the transmembrane region and at a carboxy-terminal extention. G. gallus also showed a somewhat extended region prior to the transmembrane area, as well as an extensive amino-terminal sequence not present in the other species (not shown). The shortest sequence (128 amino acids) was that of H. sapiens and the longest (318 amino acids) was that of D. rerio. The Leucoraja and Gallus Ost-beta sequences shared 43 identical amino acids, representing 24% of the entire LEE-1 sequence. Danio and Gallus shared 48 identical amino acids (Table 2). This higher number does not indicate a greater percentage of identity than the Leucoraja-Gallus comparisons, but simply reflects that Danio and Gallus had the two longest amino acid sequences among the species compared. The little skate sequence and that of human showed 23% amino acid identity, even though the time of divergence between these species is approximately 400 million years.
Table 2.
Comparison of amino acid identities for Ost-beta among species
| Ost-beta Amino Acid Homology Among Species | |||||
|---|---|---|---|---|---|
| Number of Identical Amino Acids | % Leucoraja | % Homo | % Gallus | % Danio | |
| Leucoraja-Squalus | 181 | 99% | - | - | - |
| Leucoraja-Gallus | 42 | 24% | - | 14% | - |
| Leucoraja-Danio | 36 | 20 % | - | - | 11% |
| Leucoraja-Homo | 29 | 16% | 23% | - | - |
| Homo-Gallus | 34 | - | 27% | 12% | - |
| Homo-Danio | 20 | - | 14% | - | 6% |
| Danio-Gallus | 48 | - | - | 17% | 15% |
Amino acid length for Leucoraja and Squalus 182; Homo 128; for Gallus 286; for Danio 329.
3.4 Regulation of Ost expression in vitro
In an attempt to determine if Ost expression might be regulated by components present in the cell culture environment, candidate signaling and nutritional medium supplements were tested for the ability to influence Ost expression in vitro. Medium components chosen for further scrutiny were the peptide growth factors insulin, FGF and EGF, the iron-binding protein transferrin, FBS, and the lipid mixture CDL. The effect of glutamine also was assayed as an indication of potential effects of a nutritional and medium component providing both energy and amino acid availability. Glutamine is used by elasmobranchs as a supplemental energy source by transamination to alpha-ketoglutarate and metabolism through the citric acid cycle resulting in oxidative phosphorylation and ATP generation. The general marker protein elongation factor 1-alpha was used as a control. Cells were cultured either in complete medium, or in medium in which each of the seven components indicated above were individually omitted.
Interestingly, a dramatic reduction in Ost-beta mRNA expression was seen in cells that had been cultured in the absence of the CDL lipid mixture, whereas omission of the other culture components had no significant effect (Fig. 6). The potential effect of one or more components of the CDL mixture of hydrophobic molecules was of particular interest because of the relationship of some to these to Ost transport substrates. CDL is composed of fatty acids arachidonic, linoleic, linolenic, myristic, oleic, palmitic, palmitoleic and stearic, as well as DL-alpha-tochopherol-acetate, cholesterol and emulsifiers. To determine which of these compounds might influence Ost-beta expression, as well as to verify CDL effects on Ost-beta protein expression, the effects of individual CDL components on Ost-beta expression in LEE-1 cells was assessed by immunohistochemistry. Cultures were incubated in LDF basal nutrient medium in the presence or absence of CDL components individually for 4 days and Ost-beta expression visualized in the antibody-linked assay.
Figure 6.

Expression of Ost in LEE-1 cells in vitro requires the chemically defined lipid mixture (CDL). Cells were cultured for 4 days in the full culture medium with all supplements, or under a series of conditions in which each of seven medium supplements listed below were individually omitted from the medium. Total RNA was isolated from each sample and abundance of Ost-beta mRNA estimated by RT-PCR with elongation factor 1-alpha as control. (1), all medium components present; (2), fetal bovine serum omitted; (3), insulin omitted; (4), supplementary glutamine omitted; (5), transferrin omitted; (6), CDL omitted; (7), fibroblast growth factor omitted; (8), epidermal growth factor omitted.
Absence of all CDL components led to a greatly reduced expression of Ost-beta, while incubation of cells in the presence of 0.1 uM arachidonic acid alone strongly induced Ost-beta expression (Fig. 7). Of the other CDL components, myristic acid induced Ost-beta, but to a lesser degree than that of arachidonic acid, and stearic and oleic acid also showed some stimulation of Ost-beta expression (not shown). No stimulation of Ost-beta expression was seen in the presence of exogenously added cholesterol. Because specific antibody to skate OST-alpha is not available, we looked for expression and regulation of this protein by in situ hybridization in LEE-1. OST-alpha mRNA, like OST-beta, was not detected in LEE-1 cells after incubation in the absence of CDL, but OST-alpha mRNA was obvious in a fraction of the cells in the culture after incubation with the CDL lipid mixture (Fig 8). Induction by lipids may be consistent with the report that OST alpha and beta are induced by bile acids through the FXR nuclear receptor (Landrier et al, 2006; Boyer et al., 2006).
Figure 7.
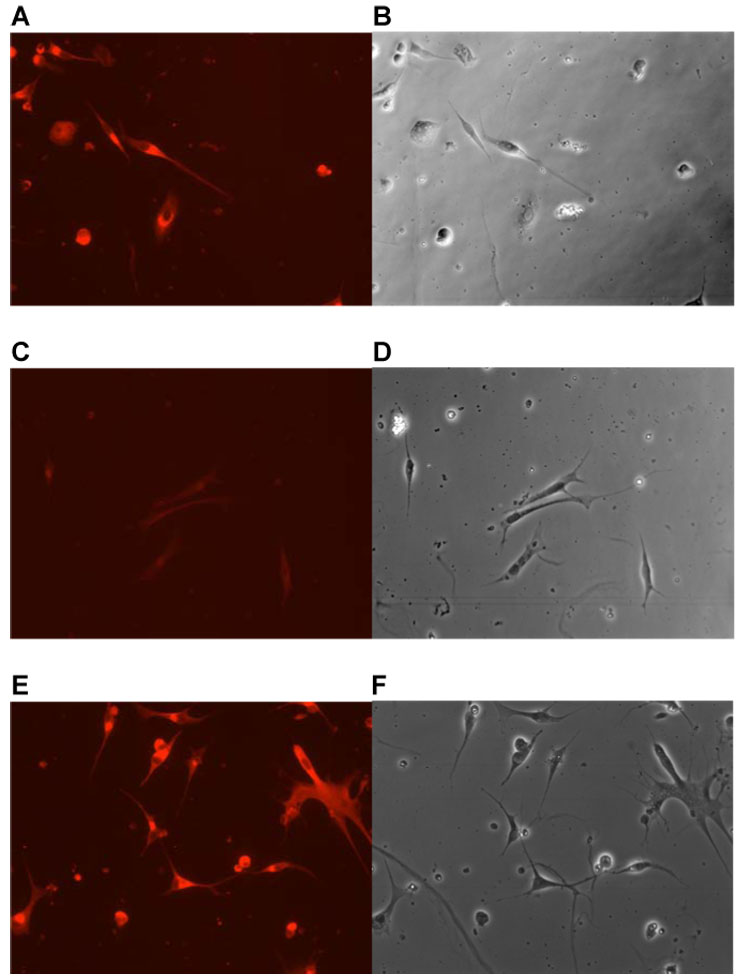
Induction of Ost-beta in LEE-1 cells by arachidonic acid. Cultures in unsupplemented, basal nutrient media were incubated in the presence or absence of CDL components individually for 4 days and Ost-beta expression visualized in the antibody-linked assay as described in Materials and Methods. Incubation of cells in the presence of arachidonic acid strongly induced Ost-beta. (A), incubation with CDL: (B), phase photomicrograph of the same field: (C), incubation without supplemental lipid: (D), phase photomicrograph of the same field: (E), incubation with arachidonic acid (0.1 uM): (F), phase photomicrograph of the same field.
Figure 8.
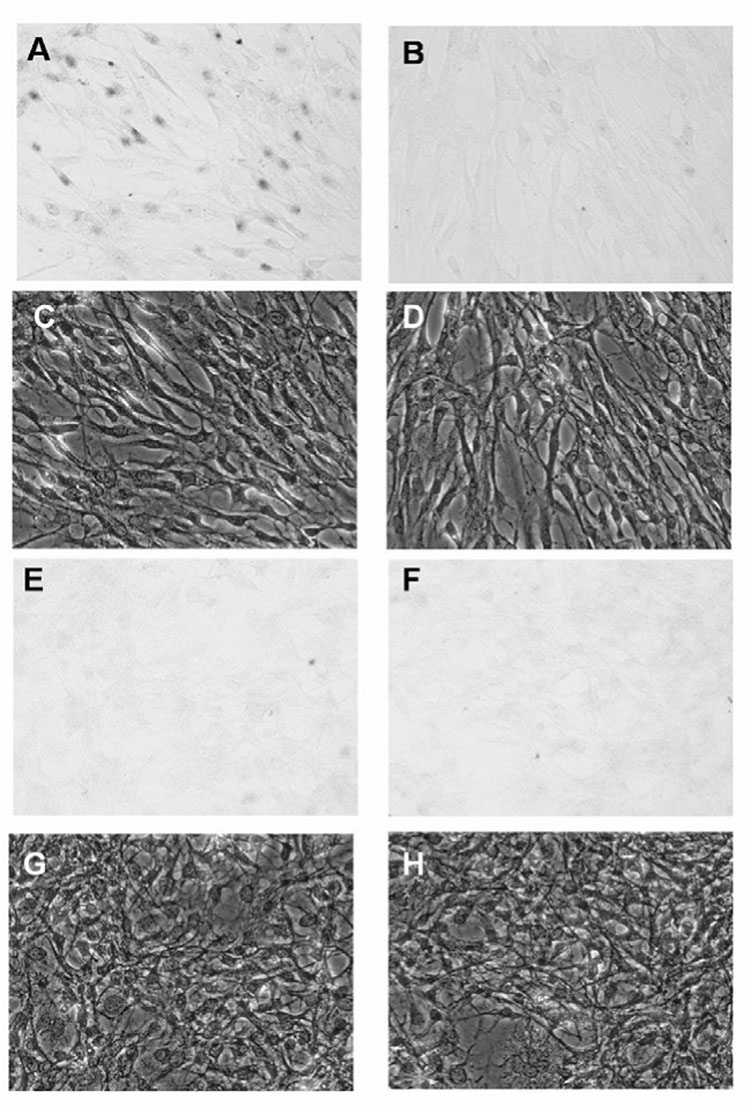
Induction of Ost-alpha in LEE-1 cells by chemically defined lipids (CDL). Cultures in unsupplemented, basal nutrient media were incubated in the presence or absence of CDL for 4 days and Ost-alpha expression visualized by in situ hybridization as described in Materials and Methods. Incubation of cells in the presence of CDL induced Ost-alpha. (A), cells incubated with CDL, assayed with antisense OST-alpha RNA: (B), cells incubated with CDL, assayed with sense OST-alpha RNA (negative control): (C), phase photomicrograph of the same field as (A): (D), phase photomicrograph of the same field as (B): (E), cells incubated without CDL, assayed with antisense Ost-alpha RNA: (F), cells incubated without CDL, assayed with sense Ost-alpha RNA (negative control); (G), phase photomicrograph of the same field as (E): (H), phase photomicrograph of the same field as (F).
4. Discussion
Ost is unique among anion membrane transport proteins in molecular structure, requiring for activity two proteins of highly different structural nature. In Xenopus oocytes, Ost mediates transport of estrone 3-sulfate, dehydroepiandrosterone 3-sulfate, taurocholate, digoxin, and prostaglandin E2. For some years, the existence of an activity functioning in mammals as the primary ileal basolateral transporter responsible for intestinal reabsorption of bile acids was postulated, but not identified (Ballatori 2005). Recent evidence is that Ost functions in this capacity in rodents and humans (Seward et al., 2003; Dawson et al., 2005; Lee et al., 2006). It has been speculated that this transporter is likely to be essential for absorption of dietary fats and vitamins, as well as bile flow and cholesterol homeostasis (Ballatori, 2005). Experiments in mammalian cell cultures have demonstrated that Ost mediates apical to basolateral vectorial transport of taurocholate and is physiologically regulated (Ballatori, 2005; Boyer et al., 2006).
Although Ost was initially identified in the little skate, very little additional work has been carried out in this animal, and no work with Ost has been reported with any other nonmammalian organisms. The skate is an elasmobranch, of the class Chondrichthyes, first appearing about 400 million years ago. Skates and rays (Superorder Batoidea) diverged from other elasmobranchs about 200 million years ago. Despite this primitive origin, the liver of the little skate exhibits many of the biochemical properties and architecture of the liver of later appearing vertebrates, including mammals. Liver functions include metabolism and clearance of endogenous anions such as bile salts, conjugated steroids, and xenobiotic lipophilic organic toxins.
Recently we derived the SAE cell line, the first from a cartilaginous fish (Parton et al., 2007), and subsequently applied the same approach to develop LEE-1. The successful derivation of cell lines from cartilaginous fish is based on the concept that culture medium formulation should be based on the physiology of the cells and a minimum of heterologous components such as FBS, rather than an adaptation process of cells to a standard medium formulation (eg., basal nutrient medium supplemented only with 10% FBS). Interestingly, the cells did not require urea in the medium, although elasmobranchs maintain a high blood urea concentration as an osmoregulator. Skate embryo cells at early stages may not require or produce high concentrations of circulating urea because they may be protected by the egg casing from a full sea water environment.
We have established that LEE-1 expresses Ost-beta at both the mRNA and protein level and confirmed that the mRNA in the cell line is a single species of the same size as that found in skate liver. Our Northern blots identified an OST-beta band of about 900 nt, somewhat smaller than the band of approximately 1 kb seen in human and rodent liver, intestine and kidney. Thus the LEE-1 cell line provides a reliable comparative model for the study of Ost in vitro. Immunolocalization in LEE-1 cells detected Ost-beta both in the plasma membrane and intracellularly, with particularly strong localization in perinuclear areas that may be endoplasmic reticulum (see Fig. 7). Similarly, Ost-beta has been found in areas other than the plasma membrane in immunolocalization studies with mammalian cells (Dawson et al. 2005; Ballatori et al. 2005; Li et al. 2007). These results suggest other functions for Ost-beta or that intercellular pools of Ost-beta are available for recruitment upon the appropriate signal. For instance, Ost-beta has been suggested to function as a chaperone, and may act in a manner similar to RAMPs, which are crucial for endoplasmic reticulum-to-Golgi translocation and plasma membrane trafficking of some receptors in mammals (Dawson et al., 2005 Bouschet et al., 2005; Parameswaran and Spielman, 2006; Hay et al., 2006, Li et al., 2007).
Ost-beta previously has been reported to be expressed in most tissues of skates, mice and humans, although expression levels vary among the tissues (Wang et al., 2001; Ballatori et al., 2005; Dawson et al., 2005) The present results demonstrate ubiquitous expression in the skate tissues examined. Skate Ost beta expression previously has been reported in liver, kidney, intestine and heart, (Wang et al., 2001). Expression in the mouse has been reported to be high in kidney and intestine, and highest levels in humans are found in the testis, colon, liver, small intestine, kidney, ovary and adrenal gland, with lower levels in a number of other tissues of both mouse and human, including brain, spleen, and heart. (Ballatori et al., 2005; Dawson et al., 2005; Li et al., 2007).
Primary sequence comparisons among L. erinacea. S. acanthias, G. gallus, D. Rerio and H. sapiens showed almost complete identity between the two cartilaginous fish, while considerable deviation from this pattern existed for the teleost D. rerio (zebrafish). The deviation is primarily the result of insertions of long amino acid regions, and is responsible for the greater length of the deduced protein, which is almost twice that of human Ost-beta. Often primary sequence comparisons among teleosts and other vertebrates are poorly related, possibly because of a recent tetraploidization event and subsequent radiation among the bony fishes. G. gallus also showed an increased length of primary structure; about 50% greater than that of the human Ost-beta.
In all cases, conserved regions were obvious in the transmembrane area, including those of shark, zebrafish and chicken; extending this information to that of these other vertebrates. A stretch of approximately 20 amino acids toward the carboxy-terminal end of the protein on the intracellular side of the membrane (Li et al., 2007) also was found to be conserved, including in chicken and zebrafish. The function of this conserved area is unknown. The role of Ost in nonmammalian and non-elasmobranch species remains to be elucidated. Evidence that the protein exists in a reasonably conserved form in both chicken and bony fish suggests transport functions that may be similar to that seen in the skate (eg., liver transport functions) or may show other critical activities similar to that seen in mammals (eg., intestinal transport functions). The tissue distributions of Ost in chicken and zebrafish are not known.
An animo acid motif of asn-arg-X-arg-lys was conserved in all species listed in Fig. 5, except human. Masking of the core arg-X-arg portion of this sequence is involved in successful transport from the endoplasmic reticulum (Margeta-Mitrovic et al., 2001). Neither of the N-linked extracellular glycosylation sites predicted for the skate protein (Wang et al.; 2001) were uniformly conserved among the five species compared. Ost-beta from L. erinacea shared 23% amino acid identity with H. sapiens, 14% with G. gallus and 11% with D. rerio. The latter, in general, showed least amino acid identity with the other species, and we have observed a similar pattern at the noncoding nucleotide level for this species (Forest et al., 2007).
Routinely, a lipid mixture was added as a supplement to the LEE-1 cells, and we found that this mixture induced Ost, indicating that the protein is under positive regulation in the usual cell culture medium. Among the individual components of this mixture, arachidonic acid was the most effective at inducing Ost-beta expression. It is interesting that Ost can transport some eicosanoids, and the principal eicosanoids (prostaglandins and thromboxanes) in humans are derived from arachidonic acid. The farnesoid X receptor (FXR) has been shown to positively regulate Ost, and is likely a critical component in signaling mechanisms controlling bile acid homeostasis (Landrier et al., 2005; Boyer et al., 2006; Lee et al., 2006; Cai et al., 2007). Our PCR evidence indicates that FXR mRNA is expressed in LEE-1 cells (not shown). Arachidonic acid has been reported to function as an FXR ligand to positively regulate FXR-mediated expression of the bile salt export pump (Zhao et al., 2004), and thus, this may be the mechanism by which the molecule stimulates expression of Ost-beta as well. However, additional studies are needed to test this possibility.
Acknowledgments
Supported by NIH grants R01-RR019732, P20-RR016463, R01-DK067214, T32-ES07026 and by National Institute of Environmental Health Sciences Center Grants ES01247 and ES03828. DB thanks Amber Miller.
Abbreviations
- Ost
organic solute and steroid transporter
- RAMP
G-protein receptor activity modifying protein
- FXR
farnesoid X receptor
- EGF
epidermal growth factor
- FGF
fibroblast growth factor
- FBS
fetal bovine serum
- DIG
digoxigenin
- BSA
bovine serum albumin
- PBS
phosphate-buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballard WW, Mellinger J, Lechenault H. A series of normal stages for development of Scyliorhinus canicula, the Lesser Spotted Dogfish (Chondrichthyes: Scyliorhinidae) J Exp Zool. 1993;267:318–336. [Google Scholar]
- Ballatori N. Biology of a novel organic solute and steroid transporter, Ost-alpha/Ost-beta. Exp Biol Med. 2005;230:689–698. doi: 10.1177/153537020523001001. [DOI] [PubMed] [Google Scholar]
- Ballatori N, Christian WV, Lee YJ, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta, a major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42:1270–1279. doi: 10.1002/hep.20961. [DOI] [PubMed] [Google Scholar]
- Barnes DW, Parton A, Tomana M, Hwang J-H, Czechanski A, Collodi P. Stem cells from cartilaginous fish. Methods in Cell Biology, 2007 doi: 10.1016/S0091-679X(08)00016-2. in press. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118:4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Trauner M, Mennone A, Soroka CJ, Cai SY, Moustafa T, Zollner G, Lee JY, Ballatori N. Up-regulation of a basolateral FXR-dependent bile acid efflux transporter, OST-alpha/OST-beta, in cholestasis in humans and rodents. Am J Physiol. 2006;290:G1124–G1130. doi: 10.1152/ajpgi.00539.2005. [DOI] [PubMed] [Google Scholar]
- Cai SY, Soroka CJ, Ballatori N, Boyer JL. Molecular characterization of a multidrug resistance-associated protein, Mrp2, from the little skate. Am J Physiol Regul Integr Comp Physiol. 2003;284:125–130. doi: 10.1152/ajpregu.00392.2002. [DOI] [PubMed] [Google Scholar]
- Cai SY, Wang L, Soroka CJ, Ballatori N, Boyer JL. An evolutionarily ancient Oatp: insights into conserved functional domains of these proteins. Am J Physiol Gastrointest Liver Physiol. 2002;282:702–710. doi: 10.1152/ajpgi.00458.2001. [DOI] [PubMed] [Google Scholar]
- Cai SY, Wang L, Ballatori N, Boyer JL. Bile salt export pump is highly conserved during vertebrate evolution and its expression is inhibited by PFIC type II mutations. Am J Physiol Gastrointest Liver Physiol. 2001;281:316–322. doi: 10.1152/ajpgi.2001.281.2.G316. [DOI] [PubMed] [Google Scholar]
- Cai SY, Xiong L, Wray CG, Ballatori N, Boyer JL. The farnesoid X receptor FXRalpha/NR1H4 acquired ligand specificity for bile salts late in vertebrate evolution. Am J Physiol Regul Integr Comp Physiol. 2007;293:1400–1409. doi: 10.1152/ajpregu.00781.2006. [DOI] [PubMed] [Google Scholar]
- Dawson PA, Hubbert M, Haywood J, Craddock AL, Zerangue N, Christian WV, Ballatori N. The heteromeric organic solute transporter alpha-beta, Ost-alpha/Ost-beta, is an ileal basolateral bile acid transporter. J Biol Chem. 2005;280:6960–6968. doi: 10.1074/jbc.M412752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forest D, Nishikawa R, Kobayashi H, Parton A, Bayne CJ, Barnes DW. RNA expression in a cartilaginous fish cell line reveals ancient 3' noncoding regions highly conserved in vertebrates. Proc Natl Acad Sci. 2007;104:1224–1229. doi: 10.1073/pnas.0610350104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenberg T, Rao A, Chen F, Haywood J, Shneider BL, Dawson PA. Regulation of the mouse organic solute transporter alpha-beta, Ostalpha-Ostbeta, by bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G912–G922. doi: 10.1152/ajpgi.00479.2005. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Helmrich A, Barnes D. Zebrafish embryonal cell culture. Methods Cell Biol. 1999;59:29–37. doi: 10.1016/s0091-679x(08)61818-x. [DOI] [PubMed] [Google Scholar]
- Landrier JF, Eloranta JJ, Vavricka SR, Kullak-Ublick GA. The nuclear receptor for bile acids, FXR, transactivates human organic solute transporter-alpha and -beta genes. Am J Physiol Gastrointest Liver Physiol. 2006;290:G476–G485. doi: 10.1152/ajpgi.00430.2005. [DOI] [PubMed] [Google Scholar]
- Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, Edwards PA. FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res. 2006;47:201–214. doi: 10.1194/jlr.M500417-JLR200. [DOI] [PubMed] [Google Scholar]
- Li N, Cui Z, Fang F, Lee JY, Ballatori N. Heterodimerization, trafficking, and membrane topology of the two proteins that constitute the organic solute and steroid transporter, Ostα and Ostβ. Biochem J. 2007;407:363–372. doi: 10.1042/BJ20070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;1:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Parameswaran N, Spielman WS. RAMPs: The past, present and future. Trends Biochem Sci. 2006;11:631–638. doi: 10.1016/j.tibs.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Parton A, Forest D, Kobayashi H, Dowell L, Bayne C, Barnes D. Cell and molecular biology of SAE, a cell line from the spiny dogfish shark, Squalus acanthias. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:111–119. doi: 10.1016/j.cbpc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Seward D, Koh A, Boyer JL, Ballatori N. Functional complementation between a novel mammalian polygenic transport complex and an evolutionarily ancient organic solute transporter, Ost-alpha-Ost-beta. J Biol Chem. 2003;278:27473–27482. doi: 10.1074/jbc.M301106200. [DOI] [PubMed] [Google Scholar]
- Sun A, Balasubramaniyan N, Xu K, Liu C, Ponamgi V, Liu H, Suchy F. Protein-protein interactions and membrane localization of the human organic solute transporter. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1586–G1593. doi: 10.1152/ajpgi.00457.2006. [DOI] [PubMed] [Google Scholar]
- Wang W, Seward D, Li L, Boyer JL, Ballatori N. Expression cloning of two genes that together mediate organic solute and steroid transport in the liver of a marine vertebrate. Proc Natl Acad Sci USA. 2001;98:9431–9436. doi: 10.1073/pnas.161099898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Yu J, Lew J, Huang L, Wright SD, Cui H. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell Biol. 2004;23:519–526. doi: 10.1089/1044549041562267. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, Trauner M. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]


