Abstract
The relationships between self-efficacy (SE), i.e., beliefs in personal capabilities, and behavioral and neuroelectric (i.e., ERN, Pe) indices of action monitoring were investigated in 40 older adults (13 male) during the completion of a flanker paradigm performed under task conditions emphasizing either accuracy or speed. SE relative to task performance during both conditions was assessed prior to each cognitive task. Results indicated that high-SE older adults exhibited larger ERN and Pe amplitudes compared to low-SE older adults under the accuracy instruction condition. Additionally, a moderating effect of SE on the relationship between ERN and post-error response accuracy was revealed in the accuracy condition, with greater ERN amplitude associated with greater post-error accuracy in the high-SE group. No significant relationships were evident between ERN and post-error accuracy in the low-SE group. Further, no significant relationships involving SE were observed in the speed condition. The findings suggest that SE may be related to neuroelectric and behavioral indices of action monitoring in older adults when task demands require greater attention to action monitoring processes.
Keywords: Cognitive Control, Cognitive Function, Error-Related Negativity (ERN), Error Positivity (Pe), Aging, Event-Related Brain Potentials (ERPs), Interference Control
1. Introduction
A considerable literature details the various aspects of cognitive decline associated with advancing age. For example, older adults exhibit deficits in performance (i.e., reaction time [RT], response accuracy) across a variety of tasks involving attention, cognition, and memory [40,46,47,49,51,58,60]. These age-related decrements in performance are disproportionately larger for tasks or task components that involve greater amounts of executive control [18,33,34] and are markedly reduced on tasks or task components that place smaller demands on the executive system [59].
1.1. Executive control
Executive control refers to a subset of processes associated with the selection, scheduling, and coordination of computational processes that are responsible for perception, memory, and action [39,43]. However, executive control processes are not unitary. Researchers have suggested that there are at least two functionally linked but dissociable systems of executive control, termed evaluative and regulative (see [11] for review). The evaluative system of executive control monitors conflict during information processing events, and signals the occurrence of conflict to centers responsible for providing compensatory, flexible, adjustments of top-down control necessary for successful online adaptations to task demands [11]. Neuroimaging research has suggested that the anterior cingulate cortex (ACC) is involved in the evaluative system by indicating when adjustments in control are warranted [37]. The regulative system exerts top-down control during ongoing information processing. That is, flexible adjustments in support are provided for task-relevant interactions within the stimulus environment, and available evidence indicates that this support is likely provided by the dorsolateral prefrontal cortex during tasks requiring inhibitory control [37].
Inhibitory control is one important aspect of executive control that is influenced by aging [27]. Inefficient inhibition results in inefficient selective attention and the intrusion of task-irrelevant information during task completion [27,34]. The introduction of task-irrelevant information can lead to both increased processing time and deficits in performance. This view has been substantiated by research documenting deficits in older adults across behavioral [34] and event-related brain potential (ERPs; [29]) measures.
A paradigm that manipulates inhibitory control requirements is the Eriksen flanker task [19]. This task requires participants to respond to target stimuli that are flanked by an array of other stimuli, which have different responses associated with them. Differences in task performance are observed when the flanking stimuli are congruent or incongruent with the target stimulus. Incongruent trials require greater amounts of inhibitory control and result in task performance deficits including longer RT and reduced accuracy due to the activation of the incorrect response elicited by the flanking stimuli, which competes with the activation of the correct response elicited by target stimuli [54].
1.2. Self-efficacy (SE)
Given that advancing age has been associated with both behavioral and cognitive impairments, the identification of modifiable lifestyle, psychological, or environmental factors associated with the preservation of executive control in older individuals is of importance for maintaining cognitive health and effective functioning. One psychosocial factor that has been associated with cognitive health and functioning during older adulthood is self-efficacy (SE; [1,2,9,10,35,53]). SE expectations reflect individuals’ beliefs in their capabilities to successfully complete courses of action [4] and are theorized to influence task choice, effort expenditure, and perseverance under aversive stimuli and failure [5]. Epidemiological research has indicated that high-functioning older adults are more likely to have higher levels of SE compared to normal or impaired functioning adults [9]. Further, SE has been positively associated with the maintenance of cognitive function over a two-year period in older adults [1], as well as being indirectly associated with cognitive performance [10,35]. Finally, SE expectations appear to play a particularly important role in self-regulatory adjustments and achievement during the completion of challenging tasks [6,13].
1.3. Action monitoring indices
1.3.1. Error-related negativity (ERN)
To date, only behavioral measures have been used to study SE benefits on cognitive function in older adults; combining behavioral and neuroelectric measures may provide for a better understanding of this relationship. Neuroelectric activity occurs continuously during the completion of cognitive tasks and ongoing neuroelectric measurement would provide a more sensitive assessment of changes in cognitive processing related to SE, which may not be manifest at a discrete, behavioral level. One ongoing cognitive process measured both behaviorally and neuroelectrically is action monitoring. Action monitoring is generally believed to be a cognitive learning mechanism used to correct an individual’s error responses during subsequent environmental interaction [55], and may be indexed by multiple neuroelectric components. One such component is the error-related negativity (ERN; [25]; or Ne; [21]). The ERN is a negative-going waveform observed in response-locked ERP averages on trials in which conflict is present, such as when an error in responding has occurred. The ERN peaks shortly after behavioral responses and is maximal over fronto-central recording sites [21,25]. The source of the ERN has been localized at or very near the caudal region of the ACC using multiple neuroimaging techniques; including dipole localization [17,57], functional magnetic resonance imaging [12], and magneto-encephalography [41]. The ERN has been theorized to reflect the detection of response conflict [11,12,61] or the transmission of a reinforcement learning signal to the ACC [30].
Consistent with the functional interaction evidenced between dissociable systems [11] and structures [26] associated with cognitive control, the ERN has been observed to be largest in situations where the recruitment of additional top-down control is required for adaptive task performance [11,21,52,61]. For example, increased ERN magnitude predicts changes in behavior that suggest increased recruitment and implementation of executive control on subsequent trials, including response slowing and increased accuracy following errors [25]. Moreover, ACC activation during task conditions that elicit response conflict predicts recruitment of additional prefrontal neural structures believed to be crucial for the implementation of control on subsequent trials [32].
ERN amplitude has been found to be smaller for older, compared to younger, adults and this difference is believed to reflect age-related degradation of the action monitoring system [3,23,42,56]. Moreover, researchers have shown increased ERN amplitude when task instructions stress accuracy over speed [20,22,25,61]. One interpretation of these findings is that the monitoring (i.e., evaluative) system flexibly adjusts according to task constraints. During tasks in which instructions stress accuracy over speed, the monitoring system is more sensitive due to the increased salience of the error in the accuracy condition [25]. An alternative explanation would suggest that this pattern of findings is due to greater attentional focus in the accuracy condition compared to the speed condition [61]. However, both of these explanations posit that changes in information processing under accuracy instructions result in increased ERN amplitude.
1.3.2. Error positivity (Pe)
A second ERP component related to action monitoring processes is the error positivity (Pe; [20,22]). The Pe is a positive-going component observed in response-locked ERP averages of incorrect responses, which is thought to be generated in the rostral region of the ACC [57]. It peaks after the ERN (about 300 ms following an error response) and is maximal over centro-parietal recording sites. The Pe has been described as a post-response evaluation of an error [16,20], an emotional (subjective) reaction to the commission of an error [22,57], or the allocation of attentional resources toward an error; similar to the allocation of attention reflected in the P3-ERP component during stimulus processing [38]. As with the ERN component, Pe amplitude is reduced in older adults compared to younger adults, suggesting a deficiency in action monitoring processes for older individuals [3]. Finally, although both the ERN and Pe are smaller in older individuals and both are associated with neural processes in the ACC; the two components are believed to be independent of each other [28].
1.4. Hypotheses
Given the relationship between SE and behavioral performance in older adults, and the association between ERP indices of action monitoring with post-error behavioral adjustments, the present study was designed to examine the association between SE and neuroelectric (i.e., ERN, Pe) and behavioral concomitants of action monitoring in older adults. With respect to task performance, it was expected that high-SE participants would respond more accurately in the accuracy condition and more quickly in the speed condition. Further, group differences in performance were predicted to be greater for incongruent, compared to congruent, trials of the flanker task, suggesting a stronger relationship between SE and performance during conditions requiring greater amounts of executive control and thereby present greater challenge to the individual. Following errors, it was predicted that the relationship between SE and behavioral measures would be stronger in the accuracy condition, with both greater response slowing and response accuracy for high-SE participants, providing additional support for increased top-down control among more efficacious individuals.
With respect to neuroelectic indices of action monitoring processes, it was predicted that high-SE participants would exhibit increased ERN and Pe amplitudes compared to low-SE participants in the accuracy condition, but not the speed condition, suggesting greater levels of self-regulatory action monitoring for more efficacious people when task instructions emphasized the salience of errors [25]. That is, increased top-down control in high-SE individuals might significantly heighten neural and behavioral adjustments following error commission to improve subsequent task performance. These adjustments would be reflected in increased ERN and Pe amplitudes as well as increased response accuracy and slowing of responses follow errors. Finally, the moderating effect of SE on the relationships between ERN, Pe, and task performance was examined to more specifically assess the associations between action monitoring processes and task performance in older adults.
2. Method
2.1. Participants
Older adults (60–73 years; M = 65.8, SD = 3.6) were recruited from a larger ongoing study at the University of Illinois to participate in this investigation. In total, 59 individuals consented to participate and all individuals reported being free of adverse health conditions, neurological disorders, any medications that influence central nervous system function, and had normal (or corrected to normal) vision based on the minimal 20/20 standard. Nineteen participants were excluded from the current analyses because they did not commit a sufficient number of errors (< 5 errors) or they did not perform above chance levels (50 % correct) in both instruction conditions of the flanker task; thus analyses were performed on 40 participants (13 males). These participants did not differ significantly from the initial sample in age, IQ, or years of education, t’s(57) ≤ 1.3, p ≥ .20.
2.2. Task
Participants completed congruent and incongruent conditions of a modified Eriksen flanker task [19]. In this task, participants were presented with an array of five arrows (<<<<<, >>>>>, <<><<, or >><>>) and were instructed to respond with a button-press to the direction of the centrally-placed target arrow. Thus, when the target arrow pointed to the left (i.e., “<”), a left thumb response was required, and when the target arrow pointed to the right (i.e., “>”), a right thumb response was required. The congruent condition had the target arrow and flanking arrows point in the same direction (i.e., <<<<< or >>>>>), and the incongruent condition had the target arrow and flanking arrows point in opposing directions (i.e., <<><< or >><>>). The arrow arrays were presented focally on a computer monitor from a distance of 1 m and each array of five arrows subtended 13.5° of the horizontal visual angle and 3.4° of the vertical visual angle when presented on the computer screen. The two congruency conditions were equiprobable and randomly ordered, with stimuli consisting of white symbols presented on a black background. Participants completed two task blocks containing 256 trials with a five-minute rest period between blocks. One block was completed under instructions to respond as accurately as possible with no regard for speed, and the other block was completed under instructions to respond as quickly as possible with no regard for accuracy. The task blocks and response instructions were counterbalanced across participants. The stimulus duration for each trial was 100 ms with a 1000 ms inter-stimulus interval. Stimulus presentation, timing, and measurement of behavioral response time and accuracy were controlled by Neuroscan Stim2 software (v 2.0).
2.3. Measures
2.3.1 Self-efficacy
Two measures were constructed to assess SE for behavioral performance under conditions that stress either speed or accuracy. These measures followed the format recommended by Bandura [4] for construction of efficacy measures and were composed of 10 items in each scale, which reflected beliefs relative to accurate completion of successively more trials on the flanker task. Thus, in the context of SE for speed, participants were asked to report their degree of confidence in completing blocks of trials as fast as possible. The first item on the scale was “I believe that I am able to accurately complete 10 out of 100 trials as fast as possible.” Each item increased by 10 trial increments so that the last item examined beliefs relative to completing 100/100 trials, again as fast as possible. Each item was scored on a Likert scale from 0% (“not at all confident”) to 100% (“highly confident”). Responses to all 10 items were summed and divided by the total number of items resulting in an efficacy score with a possible range from 0–100. The measure of SE for accuracy was constructed and scored in exactly the same manner and reflected items worded in the following manner “I believe that I am able to accurately complete × out of 100 trials without regard for speed.” Both measures had high internal consistency, α for speed = .96, α for accuracy = .96. For the purpose of subsequent analyses, participants were categorized into the high- or low-SE groups based on a median split on the SE measures.
2.3.2. Event-related potentials (ERPs)
Electroencephalographic (EEG) activity was measured using a Quik-cap (Neuro Inc., El Paso, TX) with 64 Ag-AgCl electrodes arranged in an extended 10–20 system montage, referenced to a midline electrode placed at the midpoint between Cz and CPz, while AFz served as the ground electrode. Bipolar electrooculographic activity (EOG) was recorded to monitor eye movements using Ag-AgCl electrodes placed above and below the right orbit and on the outer canthus of each eye, and all electrode impedances were kept below 10 kΩ. Neuroscan Synamps2 bioamplifiers (Neuro Inc., El Paso, TX), with a 24 bit A/D converter and +/− 200 mV input range, were used to continuously digitize (500 Hz sampling rate), amplify (gain of 10), and filter (70 Hz low-pass filter, including a 60 Hz notch filter) the raw EEG signal in DC mode (763 µV/bit resolution). EEG activity was recorded using Neuroscan Scan software (v 4.3.1).
Offline EEG processing included: eyeblink correction using a spatial filter [15], re-referencing to average mastoids, creation of response-locked epochs (−600 to 800 ms relative to behavioral response), baseline correction (100 ms time window prior to the response), band-pass filtering (1–12 Hz; 24 dB/octave), and artifact rejection (epochs with signal that exceeded ± 75 µV were rejected). Average ERP waveforms for correct trials were matched to error trial waveforms on response time and number of trials to protect against differential artifacts of the stimulus-related activity overlapping with the response-locked ERP activity [14]. ERN was quantified as the maximum negative deflection between 0–200 ms post-response [25,56] in each of these two average waveforms (error and matched-correct). Pe was quantified as the maximum positive deflection between 200–500 ms post-response [22,55] in each of the two average waveforms. Matching involved selecting individual correct trials for each participant, without replacement, that matched the response time for each of the error trials for that individual. Considering error trials are typically associated with faster RT than correct trials [23,38,61], this procedure removes artifacts that may exist in the timing of processing due to differences in response latency for correct and error trials, and results in an equal number of matched-correct trials and error trials for each individual to compare differences across accuracy conditions.
2.3.3. Response time and accuracy
Behavioral data were collected on response latency, represented by time in ms from the presentation of the stimulus, and response accuracy, reflected by the number of correct and error responses, for all trials in each task block. Errors of omission (non-responses) were categorized as incorrect responses for calculations of response accuracy, though those trials were not included in the creation of ERP waveforms due to the lack of a behavioral response. The mean number of error trials included in the ERP waveforms for high- and low-SE participants in the accuracy condition was 14 (range = 5 to 33, SD = 9.1) and 22 (range = 5 to 86, SD = 21.1), respectively. In the speed condition, the mean number of error trials for high- and low-SE participants was 34 (range = 6 to 77, SD = 22.0) and 30 (range = 7 to 70, SD = 20.5), respectively. Mean response latencies were calculated for each participant for: 1) correct trials, 2) error trials, 3) matched-correct trials (the subset of correct trials matched to specific error trials based on RT), 4) correct trials following an error trial (post-error trials), and 5) correct trials following a matched-correct trial (post-matched-correct trials). Each participant’s mean RT for correct trials following error trials was compared to his or her mean RT for correct trials following matched-correct trials in statistical analyses to provide a measure of post-error response slowing, which is a behavioral indicator of increased recruitment and implementation of top-down executive control [25,32].
2.4. Procedure
After providing informed consent in accordance with the Institutional Review Board at the University of Illinois, participants completed: a health history questionnaire, the Edinburgh handedness inventory [44], the Kaufman Brief Intelligence Test (K-BIT; [31], the Beck Depression Inventory (BDI; [8], and the Mini Mental State Exam (MMSE; [24]). The K-BIT and MMSE were administered by a trained experimenter. Participants visited the laboratory to complete behavioral and neuroelectric measures during the completion of a modified Eriksen flanker task [19]. Participants were first seated in a comfortable chair in front of a computer screen and prepared for neuroelectric measurement in accordance with the guidelines of the Society for Psychophysiological Research [48]. Participants were then given task instructions (speed or accuracy) and allowed 24 practice trials prior to the first block. Following the practice trials, participants completed the relevant SE measure to assess expectations relative to subsequent performance on cognitive task. After the completion of the first task condition, the other task condition (speed or accuracy) was completed. The protocol for this task condition was identical to the first, with participants receiving appropriate task instructions, completing 24 practice trials, and completing the relevant SE questionnaire prior to the task. Following the completion of the last block, participants were briefed on the purpose of the experiment and received $20 for their participation.
2.5. Statistical analysis
An omnibus analysis using a 2 (Accuracy: error, correct) × 4 (Site: Fz, FCz, Cz, Pz) multivariate repeated measures ANOVA [50] was conducted first to verify that these data conformed to the expected and robust topography and accuracy effects. Analyses were conducted on the four midline sites due to evidence that localizes the ERN and Pe at or near the ACC [12,17,28,41,57], which would correspond to the FCz electrode site. ERN and Pe data were analyzed separately for each instruction condition using 2 (SE: high, low) × 2 (Accuracy: error, correct) mixed-model multivariate analyses of variance (MANOVA) with repeated measures. ERN and Pe analyses did not include a Congruency factor (i.e., congruent, incongruent) due to an insufficient number of errors in the congruent condition. Behavioral data were analyzed with a 2 (SE) × 2 (Congruency) mixed-model MANOVA with repeated measures to examine group differences in the speed and accuracy of responses. The Wilks’ Lambda statistic was used for analyses with three or more within-subject levels, and post-hoc comparisons were conducted using the Bonferroni correction.
3. Results
3.1. Participant characteristics
Table 1 summarizes participants’ scores for age, MMSE, BDI, IQ, and years of education. Participant scores did not significantly differ across SE groups in the accuracy condition, t’s(38) ≤ 1.3, p’s ≥ .21, or the speed condition, t’s(38) ≤ 1.2, p’s ≥ .24. Consistent with previous research [9], high-SE individuals reported higher levels of cognitive (IQ) and emotional (BDI) functioning. However, due to the sample size (n = 40), this relationship was not significant, while previous reports with larger samples sizes (n = 1354), have observed significant effects [9].
Table 1.
Mean values (SD) for participant demographic by group in both instruction conditions.
| Measure | Speed | Accuracy | ||
|---|---|---|---|---|
| High-SE | Low-SE | High-SE | Low-SE | |
| Age (years) | 65.8 (3.5) | 65.7 (3.8) | 65.2 (3.0) | 66.2 (4.1) |
| K-BIT Composite (IQ) | 111.5 (10.2) | 109.5 (9.5) | 112.5 (9.5) | 108.6 (9.8) |
| MMSE Total Score | 27.9 (1.7) | 27.9 (1.6) | 28.3 (1.4) | 27.7 (1.8) |
| BDI Total Score | 5.1 (4.0) | 7.3 (7.2) | 5.6 (4.2) | 6.9 (7.2) |
| Years of Education | 15.2 (2.3) | 15.4 (2.8) | 15.3 (2.5) | 15.3 (2.7) |
3.1.1. Self-efficacy
There were 21 older adults in the low-SE group and 19 older adults in the high-SE group. Group sizes were unequal due to identical SE scores at the midpoint of both the accuracy SE and speed SE distributions. No theoretical justification was present for splitting the group assignments of the relevant participants, so the individuals were assigned to the same SE group in both instances, which created unequal group membership. A between-subject t test in the accuracy condition indicated a significant group difference in SE, t(38) = 9.2, p < .001, with the high-accuracy SE reporting significantly greater efficacy (M = 80.4, SD = 8.5) than the low-accuracy SE group (M = 41.0, SD = 16.9). A similar result was found in the speed condition, t(38) = 7.8, p < .001, with the high-speed SE group (M = 71.1, SD = 16.4) being significantly more efficacious than the low-speed SE group (M = 34.2, SD = 13.7). The correlation between the two SE measures was significant, r = .68, p < .001, suggesting related, but independent constructs, which was evident in the composition of the SE groups, as eight of the 40 participants in the study were categorized differently (high, low) depending on the type of SE (speed, accuracy).
3.2. Behavioral outcomes: Accuracy and response time
In the accuracy condition, analyses of response accuracy (% correct) revealed a significant Congruency effect, F(1,38) = 9.8, p = .003, ηp2 = .20, with participants performing more accurately on congruent (M = 87.3 %, SD = 12.5) compared to incongruent (M = 81.1 %, SD = 16.8) trials. No significant effects were present for SE or the interaction between SE and Congruency, although the SE findings were in the predicted direction, with high-SE participants performing more accurately than low-SE participants. A similar pattern of findings was revealed for RT, with a significant Congruency effect, F(1,38) = 96.5, p < .001, ηp2 = .72, but no effects of either SE or the interaction between SE and Congruency. Specifically, participants responded more quickly on congruent (M = 473.4 ms, SD = 73.1) compared to incongruent (M = 502.5 ms, SD = 70.5) trials.
In the speed condition, analyses of response accuracy revealed a significant Congruency effect, F(1,38) = 47.8, p < .001, ηp2 = .56, with all participants performing more accurately on congruent (M = 84.2 %, SD = 14.2) compared to incongruent (M = 76.9 %, SD = 12.8) trials. No significant effects were present for SE or the interaction between SE and Congruency. Analyses of RT revealed a similar significant Congruency effect, F(1,38) = 65.5, p < .001, ηp2 = .63, with all participants responding more quickly on congruent (M = 417.8 ms, SD = 66.8) compared to incongruent (M = 444.4 ms, SD = 71.6) trials. Further, a significant main effect of SE was present, F(1,38) = 4.7, p = .036, ηp2 = .10, with the high-SE group responding more quickly (M = 407.6 ms, SD = 73.2) than the low-SE (M = 452.0 ms, SD = 58.0) group. The interaction between SE and Congruency was not significant for RT.
3.3. Post-response behavior
To verify the hypothesized response slowing on trials following an error, an analysis of RT on error trials and correct trials following error trials was conducted. In the accuracy condition, results indicated a significant Trial effect, F(1,38) = 84.8, p < .001, ηp2 = .69, with longer RT on correct trials following error trials (post-error RT; M = 495.9 ms, SD = 85.3) compared to RT on error trials (M = 381.3 ms, SD = 61.4). Further, a significant Accuracy effect was present, F(1,38) = 15.0, p < .001, ηp2 = .28, such that post-error RT was significantly longer than RT on correct trials following matched-correct trials (i.e., post-matched-correct RT; M = 463.2 ms, SD = 72.3). Analyses of post-error response slowing and post-error response accuracy revealed no significant SE effects in the accuracy condition.
In the speed condition, a similar pattern of findings was revealed when comparing error RT to post-error RT. Specifically, a significant Trial effect was present, F(1,38) = 128.8, p < .001, ηp2 = .77, indicating significantly longer RT on correct trials following error trials (M = 448.1 ms, SD = 94.3) compared to RT on error trials (M = 343.5 ms, SD = 58.4). Analyses comparing post-error RT to post-matched-correct RT revealed a significant Accuracy effect, F(1,38) = 32.2, p < .001, ηp2 = .46, with longer RT on correct trials following errors (M = 448.1 ms, SD = 94.3) than on correct trials following matched-correct trials (M = 398.9 ms, SD = 65.1). Additionally, a significant main effect for SE was present, F(1,38) = 4.6, p < .04, ηp2 = .11, with the high-SE group responding more quickly across post-error and post-matched-correct trials (M = 397.4 ms, SD = 82.1) than the low-SE group (M = 447.1 ms, SD = 63.6). Analyses of post-error response accuracy revealed no significant SE effects in the speed condition.
3.4. ERN amplitude
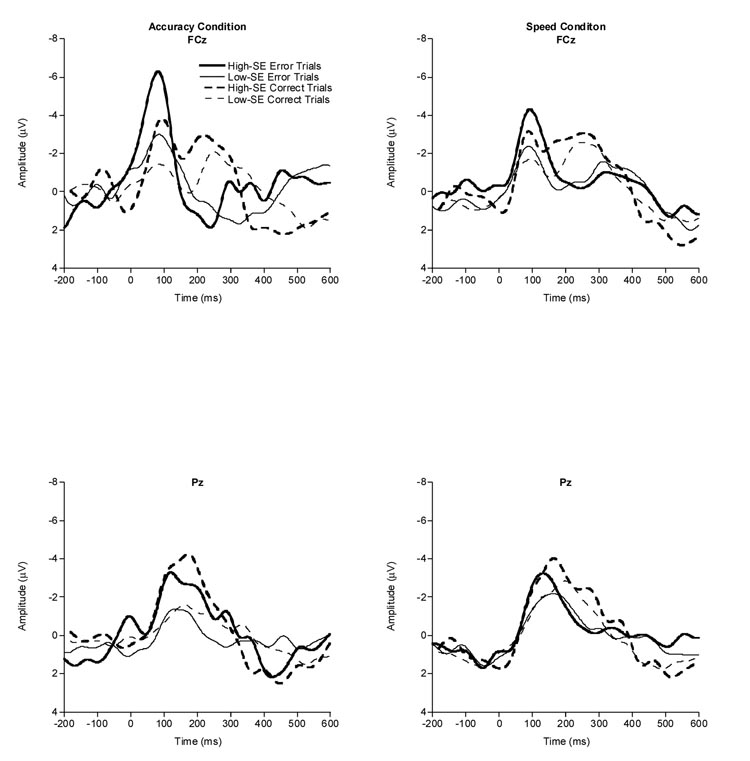
Figure 1 provides grand-averaged waveforms by SE group and instruction condition. In the accuracy condition, the omnibus analysis revealed a significant Accuracy × Site interaction, F(3,37) = 3.9, p = .017, ηp2 = .40. Post-hoc Bonferroni-corrected t tests revealed the expected significant and largest Accuracy effect at FCz, with larger ERN amplitude for error (M = −6.3 µV, SD = 4.5) compared to matched-correct (M = −4.8 µV, SD = 3.1) trials. No significant effect of Accuracy was observed at Fz, Cz, or Pz in the accuracy condition. In the speed condition, the omnibus analysis again revealed a significant Accuracy × Site interaction, F(3,37) = 4.5, p = .009, ηp2 = .27. As in the accuracy condition, post-hoc Bonferroni-corrected t tests revealed the expected significant and largest Accuracy effect at FCz, t(39) = 2.6, p = .012, with larger ERN amplitude for error (M = −4.8 µV, SD = 2.9) compared to matched-correct (M = −3.9 µV, SD = 2.4) trials. No significant effect of Accuracy was observed at Fz, Cz, or Pz in the speed condition. Thus, subsequent ERN analyses used amplitude scores from the waveforms at FCz [23,55,56].
Figure 1.
Grand averaged response-locked waveforms by SE for accuracy condition (left side) and speed condition (right side) on error and correct trials at the FCz and Pz electrode sites.
To verify the established relationship [20,22,25,61] between ERN amplitude and instruction condition (accuracy, speed), a paired-samples t test was conducted that compared ERN amplitude at FCz in the accuracy condition to ERN amplitude at FCz in the speed condition for all participants. Results indicated the expected significant effect for instruction condition, t(39) = 2.4, p = .024, with larger ERN amplitude in the accuracy condition (M = −6.3 µV, SD = 4.5) compared to speed condition (M = −4.8 µV, SD = 2.9).
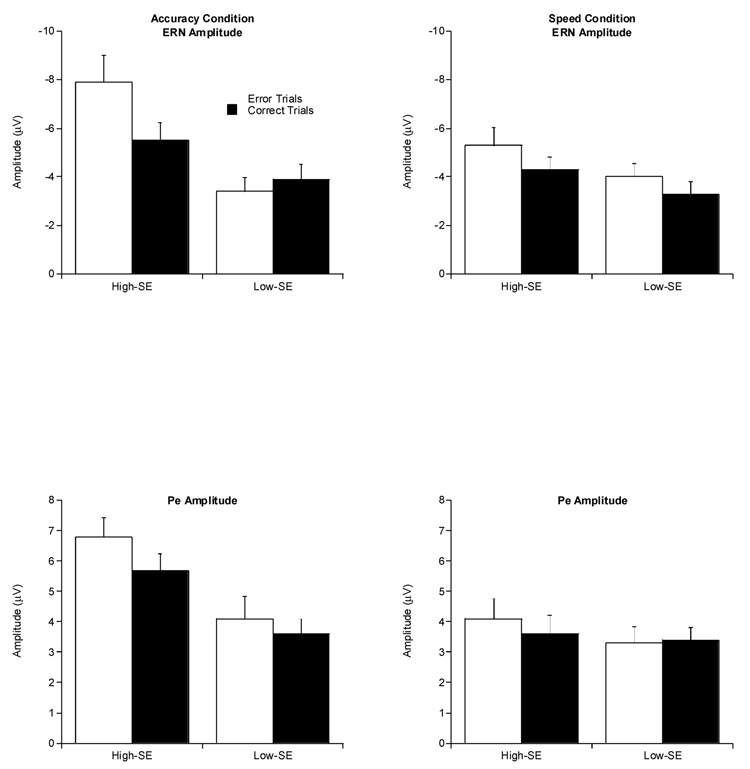
In the accuracy condition, ERN analyses revealed a significant effect for SE, F (1,38) = 12.9, p = .001, ηp2 = .3. However, this main effect was modified by a significant SE × Accuracy interaction, F (1,38) = 8.6, p < .01, ηp2 = .18, with a post-hoc Bonferroni-corrected t test, t(38) = 4.0, p < .001, indicating that ERN amplitude was significantly larger for high-SE (M = −7.9 µV, SD = 5.1) than for low-SE (M = −3.4 µV, SD = 2.4) individuals during error trials. In contrast, a post-hoc Bonferroni-corrected t test revealed no significant effect for ERN amplitude, t(38) = 2.2, p = .04, between high- (M = −5.9 µV, SD = 3.2) and low- (M = −3.9 µV, SD = 2.8) SE individuals on matched-correct trials. Finally, no significant effects or interactions were observed between SE and ERN amplitude in the speed condition. Average ERN and Pe amplitudes by SE group and instruction condition are presented in Figure 2.
Figure 2.
Average ERN and Pe amplitudes by SE for accuracy condition (left side) and speed condition (right side) on error and correct trials.
3.5. Moderating of ERN/post-error accuracy relationship
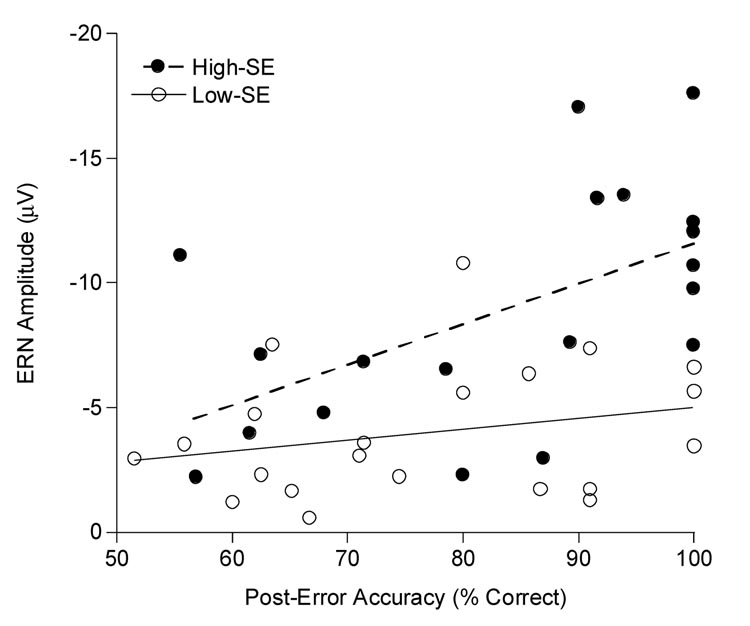
Correlations demonstrated a significant relationship between ERN amplitude and post-error accuracy in the accuracy condition, r = −.52, p < .001, with larger ERN amplitude associated with greater accuracy following error commission. Hierarchical multiple regression analyses were performed separately for both the high- and low-SE groups to determine whether the ERN relationship with post-error accuracy was moderated by SE [7]. In the first step of the regression analyses, post-error accuracy was regressed on overall response accuracy to account for the relationship between the two measures. In the second step, ERN amplitude was added to the regression equation. Table 2 provides summaries of these hierarchical multiple regressions. For the high-SE group, overall response accuracy was significant, F(1, 16) = 4.5, p < .05, R2 = .22, supporting the expected positive relationship between overall response accuracy and post-error response accuracy in the first step of the analysis. In the second step, a significant ERN effect was also observed for the high-SE group, partial-r = .60, t(16) = 3.0, p < .01, R2 change = .28, indicating that larger ERN amplitude was associated with increased post-error accuracy, independent of the relationship between overall accuracy and post-error accuracy. For the low-SE participants, the expected relationship between overall response accuracy and post-error accuracy was significant in the first step, F(1, 19) = 14.6, p = .001, R2 = .43. However, in the second step of the regression, no significant relationship was evident between ERN amplitude and post-error accuracy, partial-r = .28, t(18) = 1.2, p = .23, R2 change = .04. These findings suggest that the relationship between ERN amplitude and post-error accuracy is moderated, in part, by SE, with a positive relationship between ERN amplitude and post-error accuracy in high-SE individuals, but not low-SE individuals. Figure 3 illustrates this moderating effect of SE on the relationship between ERN amplitude and post-error accuracy.
Table 2.
Hierarchical multiple regression results to test ERN moderation of post-error accuracy (i.e., % Correct) in the accuracy instruction condition.
| Group | Variable | B | SE B | β | pr | t | Sig. |
|---|---|---|---|---|---|---|---|
| Low-SE | Step 1 | ||||||
| % Correct | .01 | .002 | .66 | .66 | 3.82 | .001 | |
| Step 2 | |||||||
| % Correct | .01 | .002 | .64 | .66 | 3.75 | .001 | |
| ERN | −.01 | .01 | −.21 | −.28 | 1.23 | .233 | |
| High-SE | Step 1 | ||||||
| % Correct | .01 | .003 | .47 | .47 | 2.19 | .042 | |
| Step 2 | |||||||
| % Correct | .01 | .002 | .42 | .50 | 2.62 | .033 | |
| ERN | −.02 | .01 | −.53 | −.60 | 3.00 | .008 | |
Figure 3.
Scatter plot for the relationship between ERN amplitude and post-error accuracy for both the high-SE and low-SE participants.
3.6. Pe amplitude
In the accuracy condition, the omnibus analysis revealed a significant Accuracy × Site interaction, F(3,37) = 13.19, p < .001, ηp 2 = .51. Post-hoc Bonferroni-corrected t tests revealed the expected significant and largest Accuracy effect at Pz, t(39) = 3.0, p = .005, with larger Pe amplitude for error (M = 5.8 µV, SD = 3.3) compared to matched-correct (M = 4.6 µV, SD = 2.5) trials. No significant effect of Accuracy was observed at Fz, FCz, or Cz. In the speed condition, the omnibus analysis revealed no significant effects for Accuracy, Site, or their interaction. Thus, subsequent Pe analyses used amplitude scores from the waveforms at Pz [22,55]. See Figure 1 for grand-averaged Pe waveforms by site and instruction condition.
In the accuracy condition, Pe analyses revealed a significant main effect for SE at the Pz electrode site, F(1,38) = 9.8, p = .003, ηp2 = .20, with the high-SE group (M = 6.8 µV, SD = 3.4) exhibiting a significantly larger Pe amplitude compared to the low-SE group (M = 4.1 µV, SD = 3.0). The lack of a significant interaction with Accuracy suggests that the high-accuracy SE group exhibited greater Pe amplitudes regardless of the behavioral response. Finally, in the speed condition, no significant effects for SE, Accuracy, or their interaction were present for Pe amplitude.
4. Discussion
The present study confirmed findings from previous research on the relationship between SE and cognitive performance in older adults and extended this literature to include neuroelectric indices of executive control. Individuals with high-SE for speed responded more quickly across all trial types than individuals with low-SE for speed. Such findings are consistent with the social cognitive perspective on relationships between beliefs in one’s capabilities and behavioral performance. Additionally, individuals with high-SE for accuracy displayed both relatively larger ERN and Pe amplitudes than individuals with low-SE for accuracy, indicating greater evaluation of error responses for high-SE participants in the accuracy condition. Conversely, the association between SE and neural indices of action monitoring was not evident in the speed condition. This relationship speaks not only to the specificity of SE beliefs [5], but also to the different motivations inherent in tasks emphasizing speed or accuracy and their reflections on the implementation of executive control as well as neural indices of self-regulatory action monitoring.
The ERN is believed to reflect the detection of conflict in the ACC [11,12,61] or the transmission of a negative reinforcement learning signal to the ACC [30], thus providing an index of the evaluative component of executive control associated with ACC activation [37]. Consequently, analysis of the ERN component provides an indication of the extent to which these evaluative processes are implemented following error commission. The present findings substantiate previous research detailing a relationship between task parameters/constraints and ERN amplitude, with increased ERN amplitude for tasks or task components focusing on accurate responses. This heightened response to errors is believed to reflect either the increased salience of an error [25] or increased attentional focus [61] when accuracy is stressed over speed.
Previous research has addressed the relationship of error salience with ERN amplitude using relatively stable personality factors [45]. Specifically, Pailing and Segalowitz [45] addressed whether error salience was enhanced more generally for all types of errors, or whether error salience increased only for errors associated with the motivational incentive. They concluded that sensitivity to incentives may be dependent upon underlying personality characteristics, but the processes reflected by the ERN “appear to operate in a very specific manner, reflecting our monitoring of errors selectively based on the incentives or consequences associated with each particular aspect of performance,” ([45], p. 94). The current pattern of findings, with increased ERN amplitude for high-SE individuals in the accuracy condition, corroborates and extends this literature.
First, the increase in ERN amplitude relationship with beliefs in one’s capability to perform a task (SE) appears to be unique to those task conditions stressing the accuracy of performance. Such tasks place greater salience and incentive on not committing errors when compared to task instructions emphasizing the speed of responding [25]. Second, SE appears to further sensitize or magnify the evaluative signal following the commission of an error above and beyond the influence of task parameters or incentives. This suggests that to best understand an individual’s reaction to an error, consideration of the role played by psychosocial factors, such as SE, may allow for a better understanding of how both behavioral and neuroelectric indices of cognition following error commission are influenced. Evidence for the association between psychological factors and post-error adjustments in behavior may be observed in the relationship between ERN and post-error accuracy for high-SE individuals. Research has described a relationship between ERN amplitude and post-error adjustments in behavior, which reflects an increase in executive control used to improve subsequent performance [25]. In this study, the relationship between ERN and post-error accuracy is present in the accuracy condition, but was moderated by SE such that the strength of this relationship was greater only in high-SE older adults. Thus, high-SE individuals show overall greater activation of action monitoring processes, as indexed by ERN. Moreover, a linear relationship was observed between post-error accuracy and ERN amplitude in these participants, with increases in post-error accuracy, an index of greater executive control, associated with increases in ERN amplitude.
The relationship between SE and Pe amplitude in the accuracy condition provides further support for the association between psychosocial factors and neuroelectric indices of action monitoring. Similar to the ERN, the Pe is also reduced in older adults [3], which substantiates the view that action monitoring processes are weakened in older adults [23]. Although debate continues as to whether the Pe reflects a predominantly cognitive [16,38] or predominantly emotional [22,57] process, all current hypotheses agree that the Pe indexes an evaluative process associated with ACC activation. In the accuracy condition, the high-SE individuals exhibited greater Pe amplitude than the low-SE individuals, regardless of the quality of the response (i.e., correct, error). Thus, these data suggest that the high-SE individuals globally implement greater online evaluative processing of their behavioral responses regardless of the quality of their responses. This increase in Pe amplitude for high-SE older adults not only suggests more evaluative processing for these individuals in the accuracy condition, but it also results in a neuroelectric profile more similar to younger adults.
Unlike the personality and mood dispositions examined in the extant literature [36,45], SE is a modifiable psychological construct. The identification of a modifiable psychological influence on cognitive processes related to action monitoring is important for older adults, given the evidence supporting an age-related degradation of the action monitoring system [3,23,42,56]. This degradation has been exhibited through relatively smaller ERN and Pe amplitudes for older adults compared to younger adults. However, those older adults with greater SE appear to selectively enhance the performance of their action monitoring system to be more similar to healthy younger adults, reflecting more effective top-down executive control. This is evidenced by larger ERN amplitudes and the positive relationship between increased ERN magnitude and increased accuracy following errors, suggesting increased recruitment and implementation of executive control on subsequent trials [25]. Whether the manipulation of older adults’ SE beliefs differentially alters the functioning of an individual’s action monitoring system and the quality of their interactions with the environment remains to be determined. For example, SE can be enhanced by the provision of efficacy information that reflects mastery and vicarious experiences, as well as social persuasion. Previous research has evidenced a relationship between SE and behavioral measures of cognitive performance [1,10,35,53], but the present study extends these findings to behavioral and neuroelectric indices of action monitoring processes.
4.1. Limitations
Although we report on interesting relationships among SE, behavioral performance, and neuroelectric indices of action monitoring, there are a number of limitations to the present study. For example, the median split assignment of individuals to SE groups as well as the cross-sectional nature of this study, limits the strength of the findings because associations may be attributable to other factors. However, participants were matched on a number of demographic factors, which helps limit other potential influences. Further, although task accuracy measures were in the predicted direction, the high- and low-SE groups in the accuracy condition did not differ in their task performance. One explanation for this finding includes the removal of participants that did not commit a sufficient number of error responses for ERP analysis. A majority of those individuals scored high on the SE scales and their inclusion in the final analysis of the behavioral data may have strengthened the group difference in task performance. Additionally, the task may not have been sufficiently sensitive to detect the group difference, and future research should implement a broader array of cognitive assessments to better assess the relationship between SE and cognitive performance. Finally, although our analyses were able to determine the extent to which SE was associated with ERN and Pe amplitudes, it is important to clarify that no causal relationship is being proposed. As noted, future efforts might consider employing true experimental designs in which efficacy expectations are manipulates to avoid the issues associated with self-selection into SE groups.
4.2. Summary
In conclusion, self-efficacy influences on action monitoring processes were examined in older adults. Our findings offer support for age-related decrements in action monitoring processes [3,23,42,56] and the position that modifiable psychological constructs may play a role in determining how an individual exerts top-down executive control following error commission [36,45]. These relationships appear to be specific to both task constraints [20,22,25,61] and psychological states that interact with those task constraints [45]. The current results suggest that self-efficacy expectations also inform how individuals respond to errors. Whether maximizing efficacy cognitions further benefits the action monitoring processes of older adults focused on the accuracy of their performances remains to be determined. How such task-specific improvements in post-error behavior might subsequently enhance cognitive well-being and quality of environmental interaction provides an intriguing basis for further exploration.
Author Note
This study was supported by grants from the National Institute on Aging (RO1 AG021188) to Charles Hillman and (RO1 AG20118) to Edward McAuley.
Footnotes
The authors do not have any conflict of interests in conducting this study or area of research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- 2.Artistico D, Cervone D, Pezzuti L. Perceived self-efficacy and everyday problem solving among young and old adults. Psychol Aging. 2003;18:68–79. doi: 10.1037/0882-7974.18.1.68. [DOI] [PubMed] [Google Scholar]
- 3.Band GPH, Kok A. Age effects on response monitoring in a mental-rotation task. Biol Psychol. 2000;51:201–221. doi: 10.1016/s0301-0511(99)00038-1. [DOI] [PubMed] [Google Scholar]
- 4.Bandura A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. doi: 10.1037//0033-295x.84.2.191. [DOI] [PubMed] [Google Scholar]
- 5.Bandura A. Social foundation of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice-Hall; 1986. [Google Scholar]
- 6.Bandura A, Cervone D. Self-evaluative and self-efficacy mechanisms governing the motivational effects of goal systems. J Pers Soc Psychol. 1983;45:1017–1028. [Google Scholar]
- 7.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 8.Beck AT, Ward CH, Mendelsohn M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 9.Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R, Finch C, Schneider E, Cotman C, McClearn G, Nesselroade J, Featherman D, Garmezy N, McKhann G, Brim G, Prager D, Rowe J. High, usual and impaired functioning in community-dwelling older men and women: Findings from the MacArthur Foundation research network on successful aging. J Clin Epidemiol. 1993;46:1129–1140. doi: 10.1016/0895-4356(93)90112-e. [DOI] [PubMed] [Google Scholar]
- 10.Berry JM, West RL. Cognitive self-efficacy in relation to personal mastery and goal setting across the life span. Int J Behav Dev. 1993;16:351–379. [Google Scholar]
- 11.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 12.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulated cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 13.Cervone D, Peake PK. Anchoring, efficacy, and action: The influence of judgmental heuristic on self-efficacy judgments and behavior. J Pers Soc Psychol. 1986;50:492–501. [Google Scholar]
- 14.Coles MGH, Scheffers MK, Holroyd CB. Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol. 2001;56:173–189. doi: 10.1016/s0301-0511(01)00076-x. [DOI] [PubMed] [Google Scholar]
- 15.Compumedics Neuroscan. Offline analysis of acquired data (SCAN 4.3 – Vol. II, EDIT 4.3) El Paso, TX: Author; 2003. [Software Manual] [Google Scholar]
- 16.Davies PL, Segalowitz SJ, Dywan J, Pailing PE. Error-negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biol Psychol. 2001;56:191–206. doi: 10.1016/s0301-0511(01)00080-1. [DOI] [PubMed] [Google Scholar]
- 17.Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- 18.DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E. General and task-specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task-switching. Neuroreport. 2001;12:2065–2071. doi: 10.1097/00001756-200107030-00054. [DOI] [PubMed] [Google Scholar]
- 19.Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonresearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 20.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A, editors. Psychophysiological brain research. vol. 1. Tilberg, the Netherlands: Tilberg University Press; 1990. pp. 192–195. [Google Scholar]
- 21.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components: II. Error processing in choice reaction tasks. Electroencephal Clin Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 22.Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 23.Falkenstein M, Hoormann J, Hohnsbein J. Changes of error-related ERPs with age. Exp Brain Res. 2001;138:258–262. doi: 10.1007/s002210100712. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 26.Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–520. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- 27.Hasher L, Zachs RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation. vol. 22. New York: Academic Press; 1988. pp. 193–225. [Google Scholar]
- 28.Herrmann MJ, Römmler J, Ehlis A, Heidrich A, Fallgatter AJ. Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe) Cogn Brain Res. 2004;20:294–299. doi: 10.1016/j.cogbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Hillman CH, Belopolsky A, Snook EM, Kramer AF, McAuley E. Physical activity and executive control: Implications for increased cognitive health during older adulthood. Res Q Exerc Sport. 2004;75:176–185. doi: 10.1080/02701367.2004.10609149. [DOI] [PubMed] [Google Scholar]
- 30.Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 32.Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 33.Kramer AF, Hahn S, Gopher D. Task coordination and aging: Explorations of executive control processes in the task switching paradigm. Acta Psychol (Amst) 1999;101:339–378. doi: 10.1016/s0001-6918(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 34.Kramer AF, Humphrey DG, Larish JF, Logan GB, Strayer DL. Aging and inhibition: Beyond a unitary view of inhibitory processing in attention. Psychol Aging. 1994;9:491–512. [PubMed] [Google Scholar]
- 35.Lachman ME, Jelalian E. Self-efficacy and attributions for intellectual performance in young and elderly adults. J Gerontol. 1984;39:577–582. doi: 10.1093/geronj/39.5.577. [DOI] [PubMed] [Google Scholar]
- 36.Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: Negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J Exp Psychol Gen. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 38.Mathewson KJ, Dywan J, Segalowitz SJ. Brain bases of error-related ERPs as influenced by age and task. Biol Psychol. 2005;70:88–104. doi: 10.1016/j.biopsycho.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multi-task performance: Part 1. Basic Mechanisms. Psychol Rev. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 40.Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain Cogn. 2001;49:277–296. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- 41.Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH. Implementation of error-processing in the human anterior cingulated cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 42.Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MGH, Holroyd CB, Kok A, van der Molen MW. A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cogn Affect Behav Neurosci. 2002;2:19–36. doi: 10.3758/cabn.2.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and Self-Regulation. Vol. 4. Advances in research and theory. New York: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- 44.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Pailing PE, Segalowitz SJ. The error-related negativity as a state and trait measure: Motivation, personality, and ERPs in response to errors. Psychophysiology. 2004;41:84–95. doi: 10.1111/1469-8986.00124. [DOI] [PubMed] [Google Scholar]
- 46.Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- 47.Park DC, Smith AD, Lauthenschlager G, Earles JL, Frieske D, Zwahr M, Gaines CL. Mediators of long-term memory performance across the life span. Psychol Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- 48.Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr., Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related-potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- 49.Polich J. Meta-analysis of P3 normative aging studies. Psychophysiology. 1996;33:334–353. doi: 10.1111/j.1469-8986.1996.tb01058.x. [DOI] [PubMed] [Google Scholar]
- 50.Rodríquez-Fornells A, Kurzbuch AR, Münte TF. Time course of error detection and correction in humans: Neurophysiological evidence. J Neurosci. 2002;22:9990–9996. doi: 10.1523/JNEUROSCI.22-22-09990.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 52.Scheffers MK, Coles MGH, Bernstein P, Gehring WJ, Donchin E. Event-related brain potentials and error-related processing: An analysis of incorrect responses to go and no-go stimuli. Psychophysiology. 1996;33:42–53. doi: 10.1111/j.1469-8986.1996.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 53.Seeman T, McAvay G, Merril S, Albert M, Rodin J. Self-efficacy beliefs and change in cognitive performance: MacArthur studies of successful aging. Psychol Aging. 1996;11:538–551. doi: 10.1037//0882-7974.11.3.538. [DOI] [PubMed] [Google Scholar]
- 54.Spencer KM, Coles MGH. The lateralized readiness potential: Relationship between human data and response activation in a connectionist model. Psychophysiology. 1999;36:364–370. doi: 10.1017/s0048577299970749. [DOI] [PubMed] [Google Scholar]
- 55.Themanson JR, Hillman CH. Cardiorespiratory fitness and acute aerobic exercise effects on neuroelectric and behavioral measures of action monitoring. Neuroscience. 2006;141:757–767. doi: 10.1016/j.neuroscience.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Themanson JR, Hillman CH, Curtin JJ. Age and physical activity influences on neuroelectric indices of action monitoring during task switching. Neurobiol Aging. 2006;27:1335–1345. doi: 10.1016/j.neurobiolaging.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 57.van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulated cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 58.Wecker NS, Kramer JH, Wisniewski A, Delis DC, Kaplan E. Age effects on executive ability. Neuropsychology. 2000;14:409–414. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- 59.West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 60.Wickens CD, Braune R, Stokes A. Age differences in the speed and capacity of information processing: I. A dual-task approach. Psychol Aging. 1987;2:70–78. doi: 10.1037//0882-7974.2.1.70. [DOI] [PubMed] [Google Scholar]
- 61.Yeung N, Cohen JD, Botvinick MM. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]