Abstract
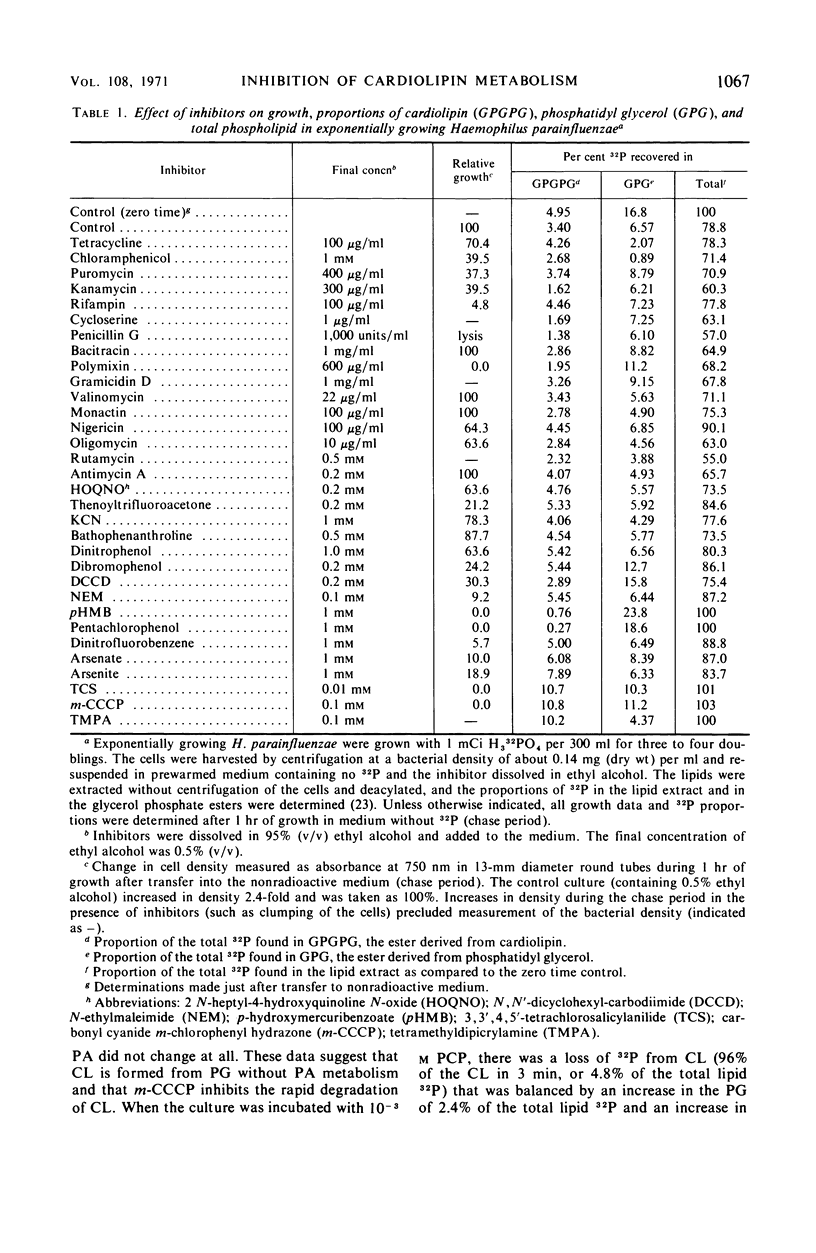
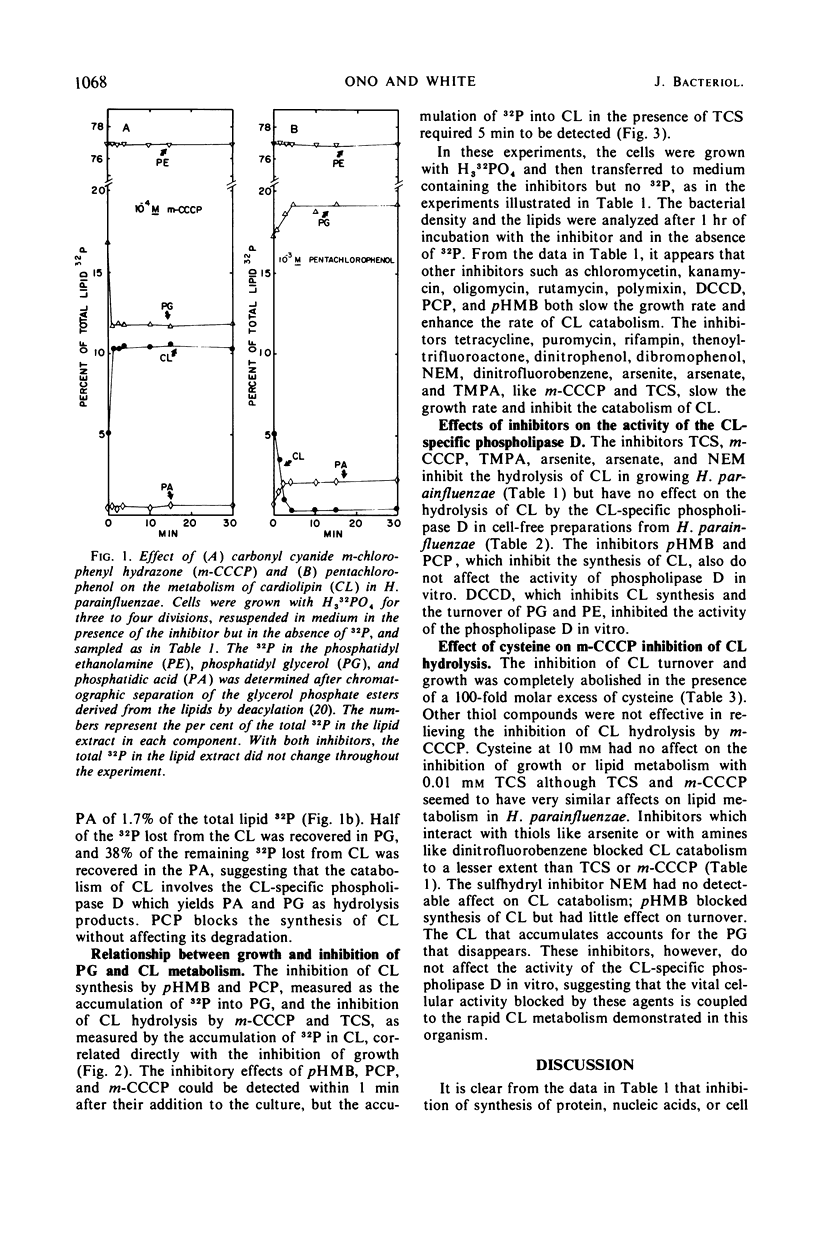
Examination of phospholipid metabolism in Haemophilus parainfluenzae with inhibitors of various cellular functions indicated that macromolecular synthesis and lipid metabolism can be dissociated at least for a short time. Two classes of inhibitors have relatively specific effects on cardiolipin (CL) metabolism. Pentachlorophenol and p-hydroxymercuribenzoate blocked CL synthesis but allowed CL hydrolysis to phosphatidic acid and phosphatidyl glycerol (PG); 3,3′,4,5′-tetrachlorosalicylanilide (TCS) and carbonyl cyanide m-chlorophenylhydrazone (m-CCCP) blocked CL hydrolysis with the stoichiometric accumulation of CL. It appeared as if TCS and m-CCCP inhibited a vital activity coupled with the hydrolysis of CL by the highly active, CL-specific phospholipase D found in this organism. Because TCS and m-CCCP are thought to act by destroying the proton gradient thereby interrupting energy-dependent transport, it is possible that a highly active portion of the cellular CL could be coupled to some phase of this process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Ballesta J. P., Schaechter M. Effect of shift-down and growth inhibition on phospholipid metabolism of Escherichia coli. J Bacteriol. 1971 Jul;107(1):251–258. doi: 10.1128/jb.107.1.251-258.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of membrane transport in Streptococcus faecalis by uncouplers of oxidative phosphorylation and its relationship to proton conduction. J Bacteriol. 1968 Dec;96(6):2025–2034. doi: 10.1128/jb.96.6.2025-2034.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Pavlasová E., Baarda J. R. A transmembrane pH gradient in Streptococcus faecalis: origin, and dissipation by proton conductors and N,N'-dicyclohexylcarbodimide. Biochim Biophys Acta. 1970;196(2):235–244. doi: 10.1016/0005-2736(70)90011-8. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meyer T. S., Moore C. E., Frank P. F. Tetramethyldipicrylamine--a new antibacterial agent. Nature. 1967 Jul 15;215(5098):312–312. doi: 10.1038/215312a0. [DOI] [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D activity in Haemophilus parainfluenzae. J Bacteriol. 1970 Jul;103(1):111–115. doi: 10.1128/jb.103.1.111-115.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D of Haemophilus parainfluenzae. II. Characteristics and possible significance. J Bacteriol. 1970 Nov;104(2):712–718. doi: 10.1128/jb.104.2.712-718.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Detection of a rapidly metabolizing portion of the membrane cardiolipin in Haemophilus parainfluenzae. J Bacteriol. 1971 Dec;108(3):1058–1064. doi: 10.1128/jb.108.3.1058-1064.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Heterogeneity of phospholipid composition in the bacterial membrane. J Bacteriol. 1970 May;102(2):508–513. doi: 10.1128/jb.102.2.508-513.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Metabolism of phospholipid 2-linked fatty acids during the release of membrane fragments from Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 Aug;103(2):329–334. doi: 10.1128/jb.103.2.329-334.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Release of membrane components from viable Haemophilus parainfluenzae by ethylenediaminetetraacetic acid-tris(hydroxymethyl)-aminomethane. J Bacteriol. 1970 May;102(2):498–507. doi: 10.1128/jb.102.2.498-507.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during bacterial growth. J Lipid Res. 1969 Mar;10(2):220–233. [PubMed] [Google Scholar]
- White D. C., Tucker A. N. Phospholipid metabolism during changes in the proportions of membrane-bound respiratory pigments in Haemophilus parainfluenzae. J Bacteriol. 1969 Jan;97(1):199–209. doi: 10.1128/jb.97.1.199-209.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodroffe R. C., Wilkinson B. E. The antibacterial action of tetrachlorsalicylanilide. J Gen Microbiol. 1966 Sep;44(3):343–352. doi: 10.1099/00221287-44-3-343. [DOI] [PubMed] [Google Scholar]
- Wuthier R. E. Two-dimensional chromatography on silica gel-loaded paper for the microanalysis of polar lipids. J Lipid Res. 1966 Jul;7(4):544–550. [PubMed] [Google Scholar]



