Abstract
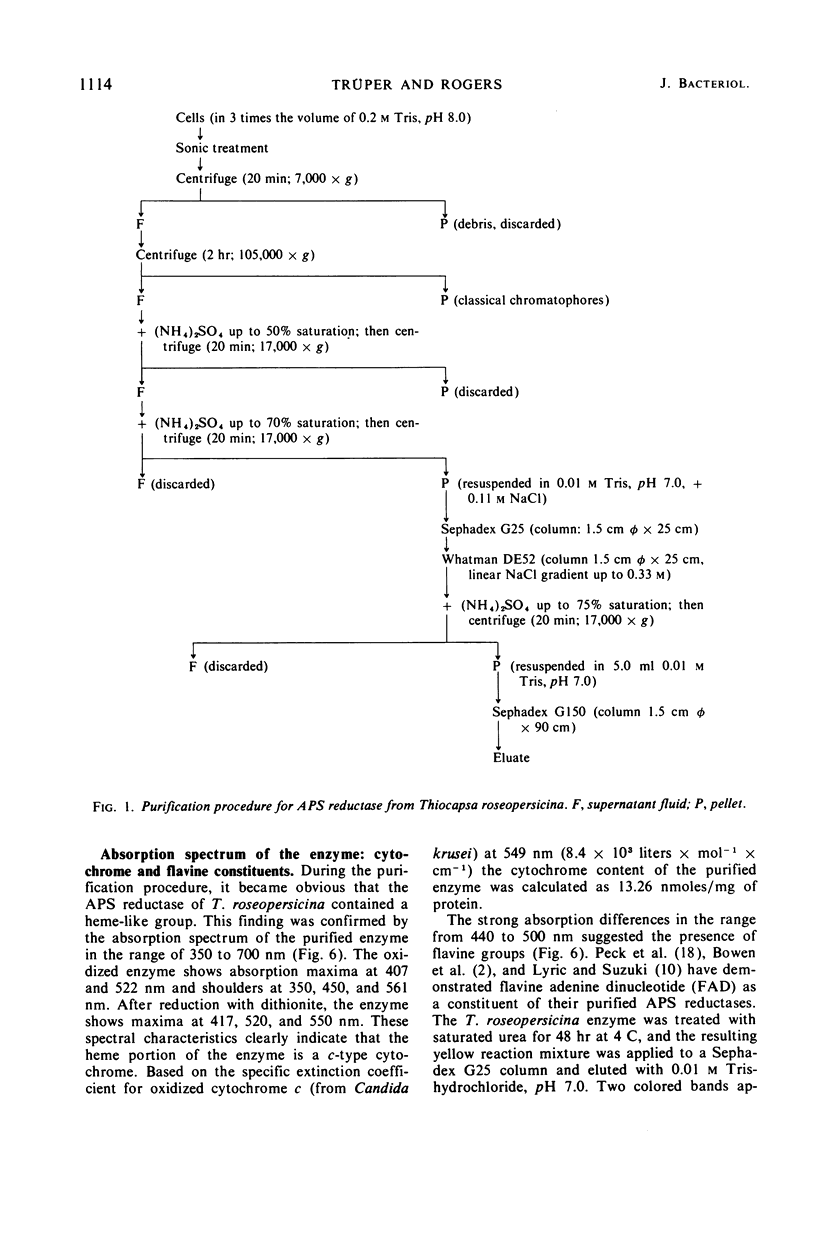
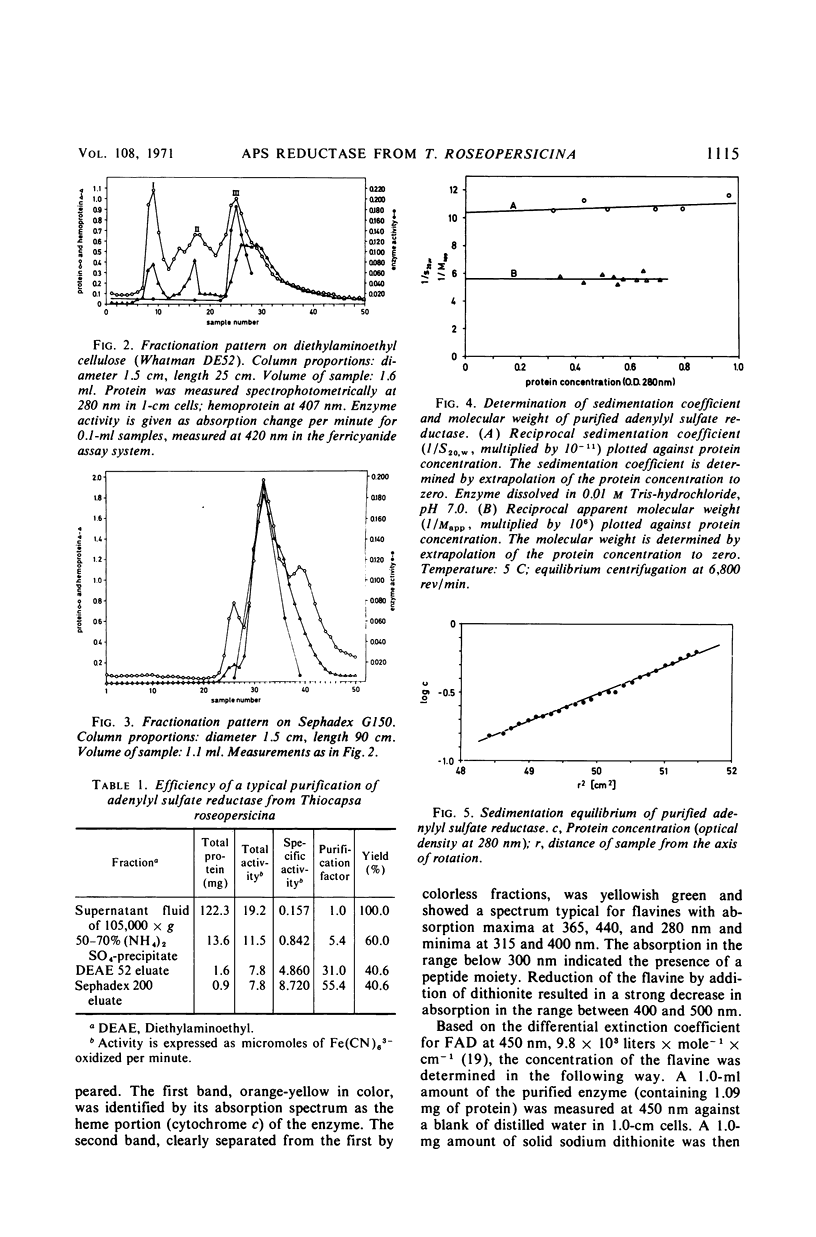
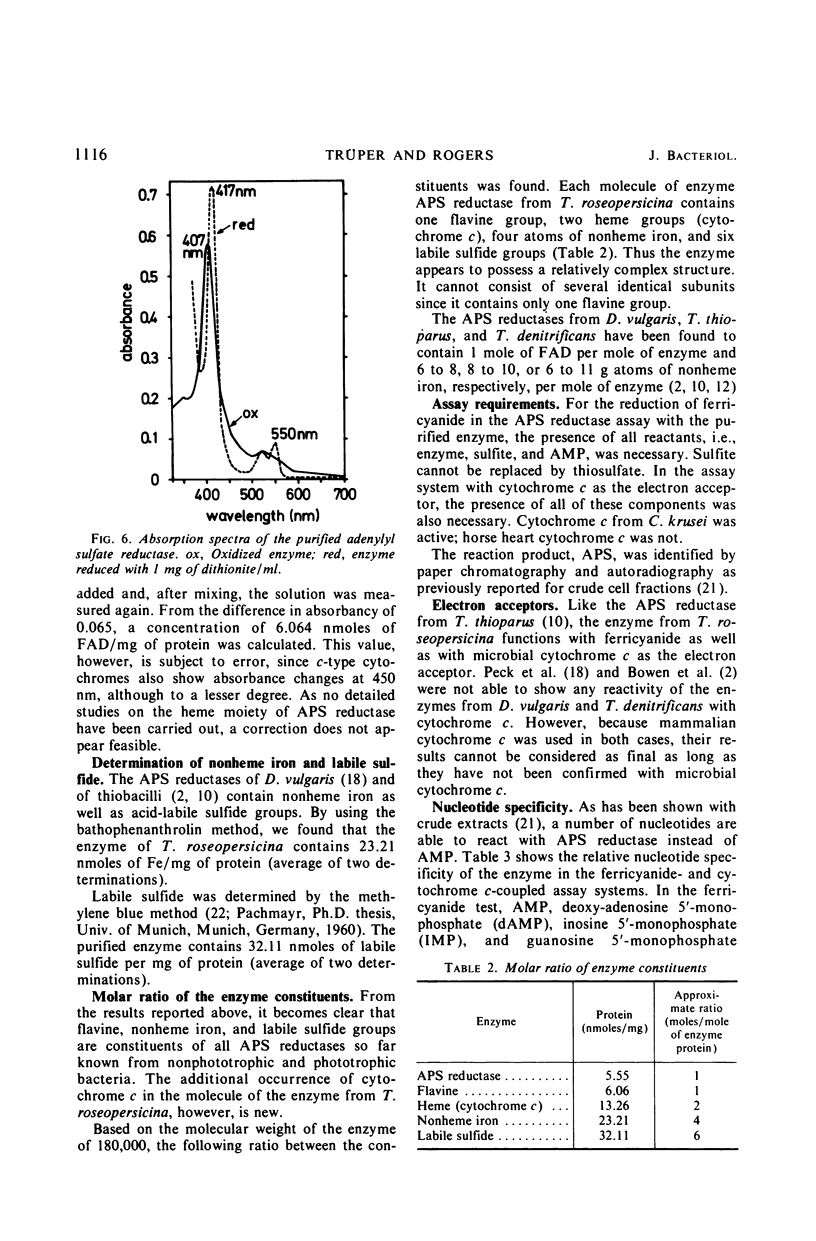
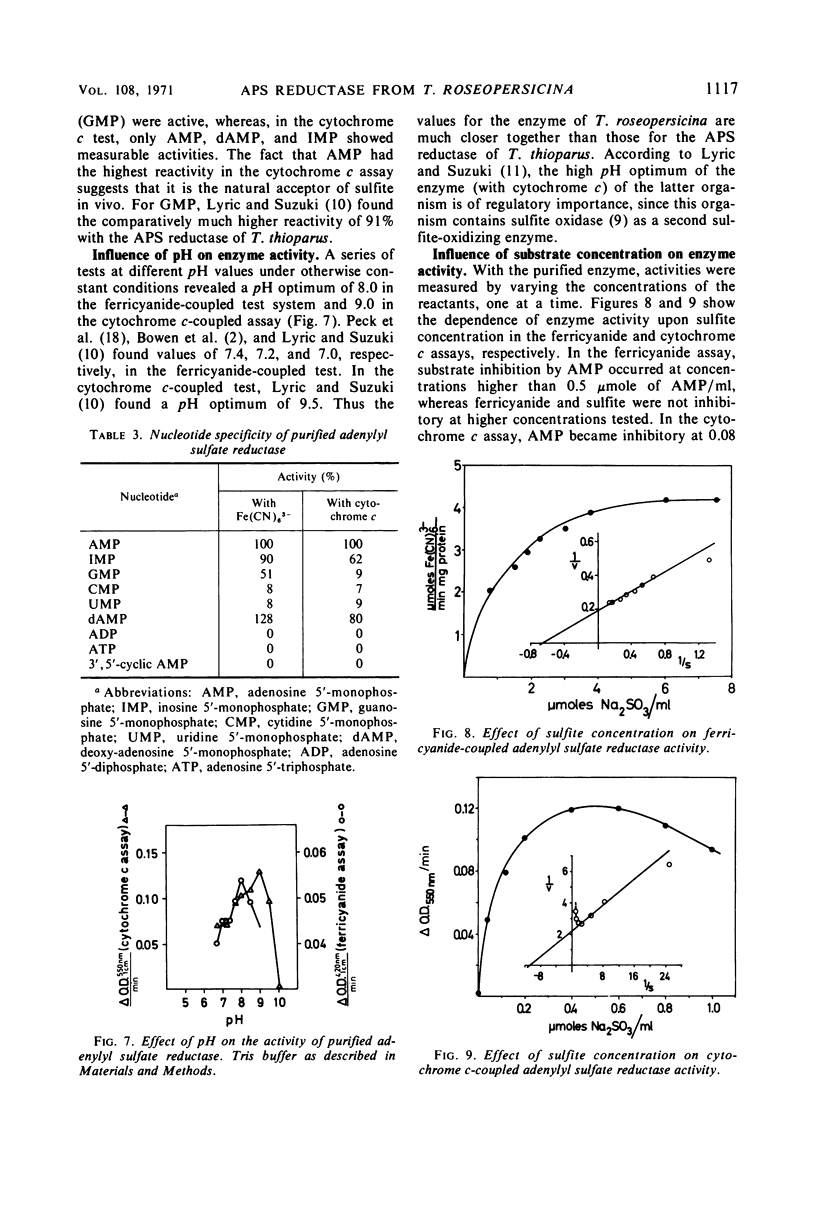
Adenylyl sulfate reductase was purified from Thiocapsa roseopersicina 60- to 80- fold, and the properties were studied. The molecular weight is 180,000. The enzyme contains, per molecule; one flavine group, two heme groups of cytochrome c character, four atoms of nonheme iron, and six labile sulfide groups. Cytochrome c and ferricyanide serve as electron acceptors. With ferricyanide as the electron acceptor, the pH optimum of the enzyme is at 8.0; with cytochrome c, the pH optimum is at 9.0. Of the nucleotides studied, adenosine 5′-monophosphate is most effective. The influence of substrate concentrations on the activity of the enzyme was studied, and the Km values for sulfite, adenosine 5′-monophosphate, ferricyanide, and cytochrome c were determined. The properties of the enzyme are compared with those of adenylyl sulfate reductases purified from sulfate-reducing bacteria and thiobacilli.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen T. J., Happold F. C., Taylor B. F. Studies on adenosine-5'-phosphosulphate reductase from Thiobacillus denitrificans. Biochim Biophys Acta. 1966 Jun 15;118(3):566–576. doi: 10.1016/s0926-6593(66)80098-x. [DOI] [PubMed] [Google Scholar]
- Broda E. The evolution of bioenergetic processes. Prog Biophys Mol Biol. 1970;21:143–208. [PubMed] [Google Scholar]
- Campbell L. L., Kasprzycki M. A., Postgate J. R. Desulfovibrio Africans sp. n., a new dissimilatory sulfate-reducing bacterium. J Bacteriol. 1966 Oct;92(4):1122–1127. doi: 10.1128/jb.92.4.1122-1127.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusanovich M. A., Bartsch R. G., Kamen M. D. Light-induced electron transport in Chromatium strain D. II. Light-induced absorbance changes in Chromatium chromatophores. Biochim Biophys Acta. 1968 Feb 12;153(2):397–417. doi: 10.1016/0005-2728(68)90083-2. [DOI] [PubMed] [Google Scholar]
- Hurlbert R. E. Effect of thiol-binding reagents on the metabolism of Chromatium D. J Bacteriol. 1968 May;95(5):1706–1712. doi: 10.1128/jb.95.5.1706-1712.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. 3. Properties of thiosulfate-oxidizing enzyme and proposed pathway of thiosulfate oxidation. Can J Biochem. 1970 Mar;48(3):355–363. doi: 10.1139/o70-058. [DOI] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. I. Survey of enzymes and properties of sulfite: cytochrome c oxidoreductase. Can J Biochem. 1970 Mar;48(3):334–343. doi: 10.1139/o70-056. [DOI] [PubMed] [Google Scholar]
- Lyric R. M., Suzuki I. Enzymes involved in the metabolism of thiosulfate by Thiobacillus thioparus. II. Properties of adenosine-5'-phosphosulfate reductase. Can J Biochem. 1970 Mar;48(3):344–354. doi: 10.1139/o70-057. [DOI] [PubMed] [Google Scholar]
- Morita S., Edwards M., Gibson J. Influence of metabolic conditions on light-induced absorbancy changes in Chromatium D. Biochim Biophys Acta. 1965 Sep 27;109(1):45–58. doi: 10.1016/0926-6585(65)90089-0. [DOI] [PubMed] [Google Scholar]
- OLSON J. M., CHANCE B. Oxidation-reduction reactions in the photosynthetic bacterium Chromatium. I. Absorption spectrum changes in whole cells. Arch Biochem Biophys. 1960 May;88:26–39. doi: 10.1016/0003-9861(60)90193-4. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr, DEACON T. E., DAVIDSON J. T. STUDIES ON ADENOSINE 5'-PHOSPHOSULFATE REDUCTASE FROM DESULFOVIBRIO DESULFURICANS AND THIOBACILLUS THIOPARUS. I. THE ASSAY AND PURIFICATION. Biochim Biophys Acta. 1965 Mar 22;96:429–446. doi: 10.1016/0005-2787(65)90561-7. [DOI] [PubMed] [Google Scholar]
- PECK H. D., Jr Enzymatic basis for assimilatory and dissimilatory sulfate reduction. J Bacteriol. 1961 Dec;82:933–939. doi: 10.1128/jb.82.6.933-939.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck H. D., Jr Energy-coupling mechanisms in chemolithotrophic bacteria. Annu Rev Microbiol. 1968;22:489–518. doi: 10.1146/annurev.mi.22.100168.002421. [DOI] [PubMed] [Google Scholar]
- TRUEPER H. G., SCHLEGEL H. G. SULPHUR METABOLISM IN THIORHODACEAE. I. QUANTITATIVE MEASUREMENTS ON GROWING CELLS OF CHROMATIUM OKENII. Antonie Van Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- Trüper H. G., Peck H. D., Jr Formation of adenylyl sulfate in phototrophic bacteria. Arch Mikrobiol. 1970;73(2):125–142. doi: 10.1007/BF00410316. [DOI] [PubMed] [Google Scholar]


