Abstract
Nitric oxide (NO) functions as a signaling agent by activation of the soluble isoform of guanylate cyclase (sGC), a heterodimeric hemoprotein. NO binds to the heme of sGC and triggers formation of cGMP from GTP. Here we report direct kinetic measurements of the multistep binding of NO to sGC and correlate these presteady state events with activation of enzyme catalysis. NO binds to sGC to form a six-coordinate, nonactivated, intermediate (kon > 1.4 × 108 M−1⋅s−1 at 4°C). Subsequent release of the axial histidine heme ligand is shown to be the molecular step responsible for activation of the enzyme. The rate at which this step proceeds also depends on NO concentration (k = 2.4 × 105 M−1⋅s−1 at 4°C), thus identifying a novel mode of regulation by NO. NO binding to the isolated heme domain of sGC was also rapid (k = 7.1 ± 2 × 108 M−1⋅s−1 at 4°C); however, no intermediate was observed. The data show that sGC acts as an extremely fast, specific, and highly efficient trap for NO and that cleavage of the iron-histidine bond provides the driving force for activation of sGC. In addition, the kinetic data indicate that transport or stabilization of NO is not necessary for effective signal transmission.
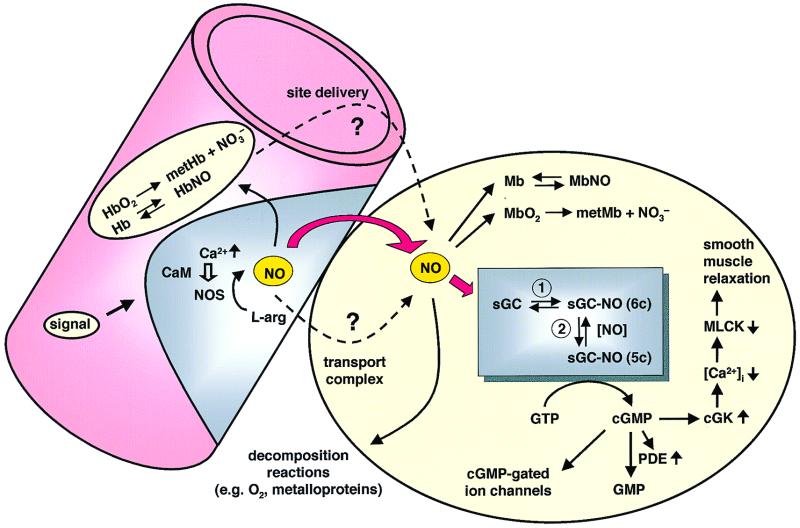
Nitric oxide (NO) typically functions in paracrine fashion; namely, NO synthesized in one cell acts on an adjacent cell to bring about a biological response such as smooth muscle relaxation involved in vasodilation (Fig. 1). NO used in signaling is synthesized by constitutive isoforms of nitric oxide synthase (NOS) that are regulated through a Ca2+/calmodulin interaction (1). Cell-specific external stimuli, such as bradykinin in endothelial cells and glutamate in neuronal tissue, increase cellular Ca2+ levels, thereby activating a specific NOS isoform. The NO signal is amplified in an adjacent cell, where it acts on the soluble isoform of guanylate cyclase (sGC) (2, 3). Once activated, sGC converts GTP to cGMP and pyrophosphate with the subsequent second messenger action of cGMP. As illustrated in Fig. 1, increases in receptor-mediated Ca2+ release in endothelial cells leads to transient activation of NOS, producing a burst of NO. NO then activates sGC in the adjacent smooth muscle, culminating in smooth muscle relaxation (vasodilation).
Figure 1.
Nitric oxide signaling in the vascular system. NO biosynthesis in the vascular endothelium is regulated by Ca2+ and calmodulin in response to external signals such as bradykinin. Upon receiving such a signal, intracellular Ca2+ is released and complexes with calmodulin leading to transient activation of NOS. The newly synthesized NO is subject to several possible fates. As indicated by the bold red arrows, free diffusion of NO into the vascular smooth muscle activates sGC. The kinetic results reported here demonstrate the feasibility of this path. Diffusion into the lumen of the blood vessel and into erythrocytes will lead to reactions with Hb and HbO2. Some evidence suggests that a nitrosylated Hb might function to deliver NO to specific tissue sites (dashed arrow) (21). It has also been suggested that small molecule carriers of NO (e.g., S-nitrosoglutathione or metal complexes) might serve as transporters. Termination of the signal via various decomposition reactions and in the smooth muscle itself via reactions with myoglobin are also shown. Based on the findings reported here, the activation of sGC is shown to proceed through two steps, with activation after step 2. Once activated, the cGMP synthesized activates a cascade of events as illustrated, leading to smooth muscle relaxation. MLCK, myosin light chain kinase; cGK, cGMP-dependent kinase; PDE, phosphodiesterase.
sGC is a heterodimeric hemoprotein. The enzyme was originally isolated from bovine lung, where the subunits were designated α1 and β1. The heme moiety is the NO binding site, which, as isolated, is ferrous and 5-coordinate with a histidine residue as the protein-derived axial ligand. By using residues 1–385 of the β1 subunit [β1(1–385)], the heme binding region has been localized to the β1-subunit with H105 as the axial heme ligand (4, 5). Steady-state analysis showed that NO binding converts the heme to a 5-coordinate nitrosyl complex in which the bond between the heme iron and the histidine has been severed. In this state, sGC is activated several hundred-fold over the basal activity. Much speculation has centered on how NO binding turns on catalytic activity. Initial studies from our laboratory on NO binding to sGC using pre-steady state kinetics revealed a complex interaction of NO with sGC (6). The kinetic traces obtained after the addition of NO were multiphasic, implying formation of at least one intermediate between the initial and final forms of the enzyme. A kinetic model was developed which, although complicated, made several predictions. The most important prediction was that binding of NO would be very fast, yielding initially a 6-coordinate ferrous-nitrosyl species that would then decay to the final 5-coordinate complex via one of two processes, one NO-dependent, one NO-independent.
Our current understanding does not answer several key signaling questions that center on how a paracrine signaling system functions when the signaling agent is short-lived, highly diffusible, and toxic. The nonspecific reactivity of NO with oxygen, metalloproteins (especially hemoproteins), and with other cellular constituents is of particular concern (7–9). At the point of generation from NOS, NO is subject to these reactions (Fig. 1). The half-life of NO in vivo, estimated to be as short as 0.1 s in the heart vasculature (10), questions the model of simple NO diffusion from the site of synthesis to the site of action in an adjacent cell (depicted by the red arrow in Fig. 1). Transport and storage using small molecules and protein thiol S-nitroso compounds (e.g., glutathione and serum albumin) has been proposed (11–17), as well as dinitrosyl iron complexes (18, 19). A recent hypothesis suggests that hemoglobin transports NO via its reactive Cys-β93 residue. In this hypothesis, when pO2 becomes low, hemoglobin undergoes an R- to T-state transition leading to release of NO from the Cys-β93 residue. Release of NO is then hypothesized to bring about vasodilation and increased oxygen delivery (20, 21).
In the results reported here, it is clear how sGC acts as an exceptionally efficient and specific trap for NO. We find that NO binds to the heme (kon > 1.4 × 108 M−1⋅s−1 at 4°C) to form a 6-coordinate ferrous-nitrosyl intermediate (Fig. 1, step 1). This intermediate then converts to the final 5-coordinate species via iron-histidine bond cleavage in an NO concentration-dependent fashion (k6c-5c = 2.4 × 105 M−1⋅s−1 at 4°C) (Fig. 1, step 2). The NO-dependence of the second step is a substantial finding because we also show that this is the key step in catalytic activation and, consequently, in signaling through this pathway. The near diffusion-limited initial interaction permits sGC to be an extremely fast, sensitive, and specific receptor for NO, in effect obviating the need for NO site-to-site transport, whereas the secondary interaction permits a sliding scale of signal transduction in response to changing concentrations of NO.
Materials and Methods
Expression and Purification of sGC and β1(1–385).
sGC was purified from Sf9 cells infected with recombinant baculoviruses as described (22). β1(1–385) was expressed in Escherichia coli and was purified as described (4) with the following modifications: E. coli BL21 (pLysS) containing the β1(1–385) expression plasmid was grown in an expression media (4.5% yeast extract/17 mM KH2PO4/72 mM K2HPO4) instead of modified Terrific Broth.
Preparation of NO Solutions.
NO gas (Matheson) was further purified by bubbling through a saturated KOH solution. Buffer (50 mM Hepes, pH 7.4/50 mM NaCl/10 ml) was made anaerobic in a tonometer by 20 cycles of vacuum and argon replacement using an oxygen-scavenged gas train. A saturated NO solution was made by bubbling NO through the 10 ml of anaerobic buffer for 30 min at room temperature in a tonometer equipped with a Teflon septum (Aldrich). The needle holes in the Teflon septum were sealed with grease to prevent leakage of NO and oxygen. The saturated NO solution was diluted to appropriate concentrations as follows: 4 ml of buffer in a 10-ml syringe with a three-way stop valve attached was made anaerobic by bubbling argon for 10 min at room temperature. An aliquot of the saturated NO solution was removed from the tonometer by using a gas-tight syringe (Hamilton). Before mixing the saturated NO solution with the 4 ml of buffer in the 10-ml syringe, the gas phase in the 10-ml syringe was removed to prevent the equilibrium of NO between liquid and gas phases. This permits reliable and reproducible dilutions.
NO Concentrations.
The concentration of NO solutions was determined by forming the NO complex of β1(1–385) and is described elsewhere (Y.Z., D.P.B., and M.A.M., unpublished work). Because the Kd for NO binding to β1(1–385) is in the picomolar range, the NO concentration is equal to the final β1(1–385)-NO concentration. In brief, NO binding shifts the β1(1–385) λSoret from 431 to 399 nm. The difference spectrum shows a peak at 397 and a trough at 434 nm. NO concentrations were determined by measurement of the ΔA397 − ΔA434.
Stopped-Flow Analysis of NO Binding to sGC and β1(1–385).
Protein samples in 50 mM Hepes (pH 7.4) and 50 mM NaCl were made anaerobic in a tonometer by 10 cycles of vacuum followed by argon replacement using an oxygen-scavenged gas-train. The stopped-flow apparatus was made anaerobic by filling the instrument for 12 hours with anaerobic buffer containing protocatechuate dioxygenase and protocatechuic acid as an oxygen scavenging system (23). The sGC solution was mixed with NO solutions at 4°C in the stopped-flow instrument, and kinetic traces were recorded at 431, 399, and 420 nm. NO binding to β1(1–385) was carried out the same way.
Determination of the Activity of the 6-Coordinate sGC-NO Complex.
sGC [1 μM, in 50 mM Hepes (pH 7.4) and 50 mM NaCl, 15 μl] was rapidly mixed with buffer (15 μl) containing GTP (3 mM), Mg2+ (10 mM), and NO in a KinTek Rapid Quench Flow device (model RQF-3; KinTek, State College, PA) at 4°C. The two buffer drive syringes contained argon-sparged 50 mM Hepes (pH 7.4) and 50 mM NaCl, and the quench syringe contained 40 mM HCl. The reaction loop volume was 60 μl. The sGC solution was made anaerobic in a Reacti-vial (Pierce), was transferred to a gas-tight syringe, and was mounted on the Rapid Quench Flow device. A stock solution of NO (2 mM) was prepared by bubbling buffer (1 ml) in a Reacti-vial with argon (10 min) and then with NO (15 min). NO was diluted from a freshly prepared stock solution to the required concentration in an anaerobic substrate solution in a 5-ml gas-tight syringe. The quenched reaction mixture was treated with 0.4 ml Zn(OAc)2 (125 mM) followed by 0.5 ml Na2CO3 (125 mM). cGMP was quantified by ELISA (Biomol, Plymouth Meeting, PA). The specific activity of sGC at 4°C was 1.3 and 108 nmol/min/mg in the absence and presence of NO derived from DEA-NONOate (24), respectively.
Results
NO Binding to sGC.
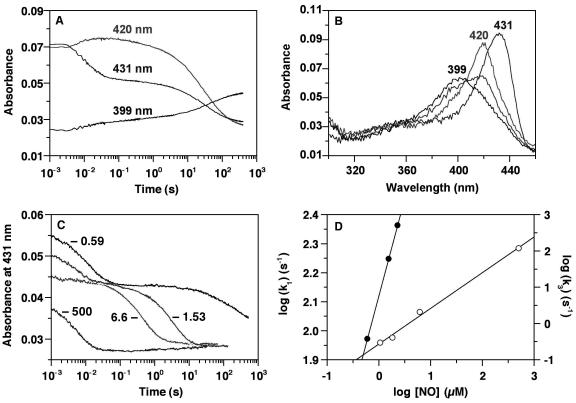
Binding of NO to sGC was examined by stopped-flow spectroscopy (Fig. 2A). The reaction was carried out at 4°C and at low concentrations of both NO and protein so that the binding process would not be complete in the instrument dead-time (3-ms period between mixing and the start of data accumulation). Rapid mixing of sGC with NO leads to formation of a 6-coordinate ferrous-nitrosyl complex (λSoret at 420 nm) (25) within ≈30 ms (Fig. 2A). This complex converts to a 5-coordinate NO complex (λSoret at 399 nm) as the bond between the heme iron and the axial histidine breaks. Three kinetic phases were observed at low NO concentrations (Fig. 2B). The first phase was fast, depended on the concentration of NO, and represents initial NO binding. The second phase (≈0.1–10 s) was small (<10% of the total), varies between preparations, and is probably attributable to small quantities of partially denatured enzyme. The third phase, which leads to the 5-coordinate NO-complex, clearly depended on the concentration of NO (Fig. 2 C and D). To facilitate analysis, we fit the traces to three parallel exponential processes. This gave a calculated second-order rate constant for NO binding (phase 1) to sGC of 1.4 × 108 M−1⋅s−1 at 4°C. However, under the conditions of the experiment, NO binding is in fact second order, and nearly 50% of the reaction occurs within the dead time of the instrument (≈3 ms), even at concentrations as low as 0.57 μM NO (sGC = 0.6 μM). For a second-order reaction, such an analysis will underestimate the binding rate constants; thus, 1.4 × 108 M−1⋅s−1 is a lower limit for this reaction at 4°C. Table 1 shows that at temperatures of 20–25°C the binding of NO to other hemoproteins is at least 10-fold slower than to sGC at 4°C. Thus, at the same temperature, the true differences in kon are even larger.
Figure 2.
Stopped-flow analysis of NO binding to heterodimeric sGC. (A) All concentrations are postmixing. sGC (0.6 μM) and NO (0.57 μM) were mixed anaerobically in a stopped-flow spectrophotometer (Hi-Tech Scientific, Salisbury, U.K.) at 4°C. Absorbance changes at 399, 420, and 431 nm are shown. (B) Spectra are shown of the ferrous sGC recorded before the reaction with NO (λSoret at 431 nm), of the 6-coordinate NO complex intermediate immediately after mixing sGC with NO (λSoret at 420 nm), and of the 5-coordinate NO complex recorded 5 min after initiating the reaction (λSoret at 399 nm). A spectrum of a mixture of 6- and 5-coordinate NO complexes during conversion to the 5-coordinate NO complex is shown and also shows the isosbestic point at 406 nm. The sGC concentration was 0.6 μM, and the NO concentration was 0.57 μM. Spectra were recorded at 200 nm/s. (C) The effect of NO concentration on the overall reaction was examined as follows: sGC (0.47 μM) was mixed anaerobically with 0.59, 1.53, 2.28 (data not shown for clarity), 6.6, and 500 μM NO at 4°C, and absorbance changes at 431 nm are shown. (D) The data in C were fit to three consecutive exponential processes. kobs obtained from the first phase (k1), which represents NO binding to sGC heme (filled circles), and that for the third phase (k3), which represents conversion of the 6- to the 5-coordinate ferrous-NO complex (open circles), are plotted against the NO concentration. For the k3 points, the NO concentrations were corrected by the amount that is bound to the heme in the first phase: i.e., the sGC concentration.
Table 1.
Rate constants for NO binding to hemoproteins
| Hemoprotein | kon, M−1·s−1 | koff, s−1 | Temperature, °C | Reference |
|---|---|---|---|---|
| sGC | >1.4 × 108 | n.d. | 4 | This work |
| n.d. | 8.2 × 10−4 | 25 | 38 | |
| n.d. | 1.7 × 10−2 | 37 | 22 | |
| β1(1–385) | 7.1 ± 2 × 108 | n.d. | 4 | This work |
| Hb(T) | 1.8 × 107 | 4.0 × 10−3 | 20 | 39 |
| Hb(R) | 1.8 × 107 | 5.0 × 10−5 | 20 | 39 |
| Mb | 1.7 × 107 | 1.2 × 10−4 | 20 | 9 |
| OxyMb* | 3.7 × 107 | n.a. | 25 | 40 |
n.d., not determined; n.a., not applicable.
The rate constant cited for oxymoglobin is a reaction rate constant, not a binding rate constant.
The conversion of the 6-coordinate intermediate to the final 5-coordinate nitrosyl complex was at least three orders of magnitude slower than the initial binding step, as evidenced by the rate constants for these processes [2.4 ± 0.2 × 105 M−1⋅s−1 vs. >1.4 × 108 M−1⋅s−1, respectively (Fig. 2D)]. Both of these rates are first order in NO; however, they do not extrapolate to same point in Fig. 2D. This is principally attributable to the contribution (≈50 s−1) of the back reaction in the fast phase. The conversion of the 6-coordinate intermediate to the 5-coordinate nitrosyl complex depended on NO concentration (Fig. 2D), indicating the presence of a second binding site for NO on sGC. Binding of NO at this site is not an absolute requirement for cleavage of the iron-histidine bond because conversion of the 6- to the 5-coordinate NO complex occurs with substoichiometric NO, although much more slowly (0.0087 s−1).
sGC Activity Measurements.
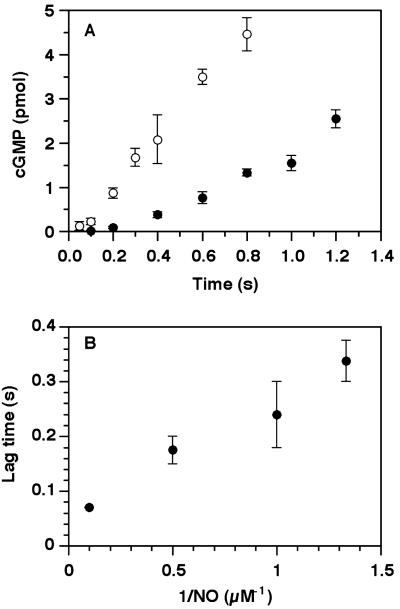
Observation of a relatively stable 6-coordinate ferrous-nitrosyl intermediate provided the opportunity to determine whether cleavage of the iron-histidine bond is necessary for activation. The activity data from rapid-quench reactions (Fig. 3) show that the intermediate is not activated. Therefore, activation occurs either simultaneous with or immediately after iron-histidine bond cleavage. Furthermore, when sGC was rapidly mixed with substrate (Mg2+GTP) and NO, there was a lag before product appearance. The length of this lag was inversely dependent on NO concentration (Fig. 3B). Although there are several possible explanations for the lag, our evidence strongly suggests that it is caused by the conversion of the 6-coordinate species to the 5-coordinate NO complex. The lag cannot be attributed to the time required for initial binding of NO to the heme because, even with substoichiometric amounts of NO, the initial binding process was essentially complete before the first sample was quenched (Fig. 3B). Additionally, the binding of NO to sGC is not significantly changed by the presence of substrates: When binding of NO to sGC in the presence of 1.5 mM GTP and 5 mM MgCl2 was monitored in the stopped-flow, the kinetics of NO binding were the same as those without substrates (data not shown). The final activated rate is independent of the NO concentration, as long as [NO] > [sGC]. For example, at 37°C, where the rate of activation is very rapid, the enzyme activity after 1 min of activation was constant over the range of 0.1–100 μM DEA-NONOate (sGC = 10 nM) (not shown).
Figure 3.
Determination of the activity of the 6-coordinate sGC-NO complex intermediate. (A) sGC [1 μM, in 50 mM Hepes (pH 7.4) and 50 mM NaCl, 15 μl) was rapidly mixed with 15 μl of buffer containing GTP (3 mM), Mg2+ (10 mM), and NO in a Kintek Rapid Quench Flow device at 4°C and were quenched at appropriate times with 40 mM HCl. Data for NO concentrations after mixing at 0.75 μM (filled circles) or at 10 μM (open circles) are shown. The sGC heme concentration was 0.5 μM after mixing. Each point is the mean of the cGMP values determined for three measurements ± SD. (B) The dependence of lag time on NO concentration. The lag time is the time before which no cGMP was detectable (limit, 0.15 pmol). Each point is the average value from two independent experiments. The P values determined for the lag times using ANOVA were <0.04 for each nonadjacent pair of reciprocal NO concentrations.
The overall conclusion from this set of experiments is that catalysis does not occur until the 5-coordinate species is formed. Indeed, at the lower concentration of NO shown in Fig. 3A, product accumulation is slower than at the higher concentration because less sGC has converted to the active form within the time of the activity experiment (compare with Fig. 2C).
NO Binding to the Heme Domain β(1–385).
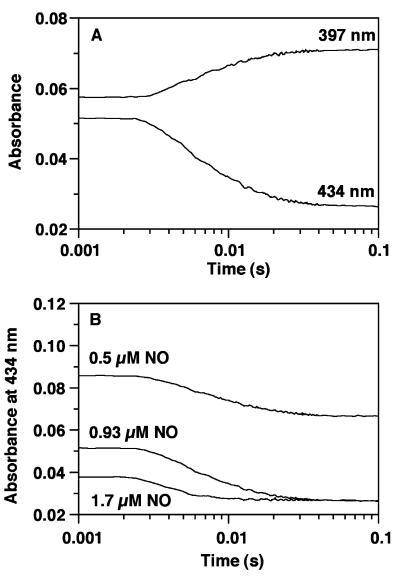
Examination of NO binding to β(1–385) by using the same stopped-flow spectroscopic methods described above showed that β1(1–385) was converted directly to the 5-coordinate ferrous-nitrosyl complex (Fig. 4A) with no detectable intermediate species. Stopped-flow traces (Fig. 4B) were simulated according to the second order process A + B → C, where A = β1(1–385), B = NO, and C = β1(1–385)-NO. From this analysis, kon is estimated to be 7.1 ± 2 × 108 M−1⋅s−1 at 4°C. It should be noted that >60% of the reaction has occurred in the dead-time of the stopped-flow. This rate constant is similar to that for heterodimeric sGC, indicating that binding of NO was not greatly affected by the absence of the catalytic domain.
Figure 4.
Stopped-flow analysis of NO binding to β1(1–385). (A) All concentrations are postmixing. β1(1–385) and NO were mixed anaerobically in a stopped-flow (Hi-Tech Scientific) at 4°C. After each experiment, a final spectrum was recorded to measure the total absorbance change at either 397 nm or 434 nm. The stopped-flow traces shown were obtained at 434 and 397 nm when both β1(1–385) and NO were 0.93 μM. (B) Nitrosyl complex formation at different NO concentrations (0.5, 0.93, 1.7 μM), β1(1–385) = 0.93 μM. Stopped-flow traces were simulated according to the second order process A + B → C, where A = β1(1–385), B = NO, and C = β1(1–385)-NO. From this analysis, kon is estimated to be 7.1 ± 2 × 108 M−1⋅s−1 at 4°C. It should be noted that >60% of these reactions have occurred in the dead-time of the stopped-flow instrument.
Discussion
NO is highly reactive under the biological conditions in which it is generated. Hence, any use of NO in biology must have evolved a mechanism to bring about the desired function while avoiding toxicity. Tight control over the biosynthesis of NO is required and is known to be accomplished by a rigorously regulated switch involving Ca2+ and calmodulin (Fig. 1) (1). A key question is: Does NO get to target cells by free diffusion or is it trapped/converted to a transportable form that allows for delivery either to adjacent cells or some distant site? These possibilities are outlined in Fig. 1.
The on-rate of (kon) > 1.4 × 108 M−1⋅s−1 for NO binding to sGC at 4°C is the largest ever determined for a hemoprotein and approaches a diffusion-limited rate (Table 1), leading us to conclude that there is no kinetic requirement for a transport system, at least when signaling involves a directly adjacent cell. Current transport hypotheses for which there is some evidence involve thiol modification (11, 15, 20, 21). However, thiols do not react directly with NO. S-nitroso bond formation in aqueous solution occurs via reaction with N2O3, an intermediate in the solution decomposition of NO to NO2−. These solution reactions and the subsequent bimolecular reaction of N2O3 will be slow compared with the reaction of NO with sGC. O2 binding to sGC is not detectable (26), in contrast to other ferrous hemes with axial histidine ligation, such as hemoglobin and myoglobin. Thus, sGC has evolved to distinguish NO from O2, and can exclude binding of O2, even when the concentration of O2 is much greater than that of NO.
The final species formed in the stopped-flow is the same final species that has been previously observed and characterized under steady-state conditions (22, 26–29). As mentioned earlier, we had previously observed multiphasic behavior in the binding of NO to sGC that was suggestive of at least one intermediate species (6). Recently, by using EPR and absorbance spectroscopy, this complex was definitively assigned as a 6-coordinate ferrous-nitrosyl adduct (25). The intermediate that we observed is the same as that seen by Makino et al. (25). Using rapid/quench methods, we were able to show that this 6-coordinate intermediate does not have elevated sGC activity; it is only the final 5-coordinate species that is activated. Therefore, breakage of the heme-His bond is coincident with activation (Fig. 3A).
Our kinetic results show that conversion of the 6-coordinate intermediate to the final 5-coordinate species is also dependent on NO and that this conversion step is the “slow” step in the activation of the enzyme (Fig. 2D). However, at 37°C, the enzyme activation step (Fig. 1, 6c–5c) takes <100 ms in the presence of low micromolar concentrations of NO, placing the conversion in a physiologically significant time frame. Furthermore, and most importantly from the signaling perspective, the observation that conversion of the 6-coordinate NO complex intermediate to the 5-coordinate activated NO complex of sGC depends on NO concentration suggests a novel mechanism of sGC regulation: The NO concentration will not only control how much enzyme is activated at any given time, but also how fast the enzyme is activated (Fig. 3).
The dependence of the conversion of the 6-coordinate intermediate to the final 5-coordinate activated species on NO is perhaps the most intriguing of the results reported. The possibilities for the dependence of this conversion on NO concentration include: (i) a second (non-heme) NO binding site that interacts with the heme-nitrosyl to assist in loss of proximal coordination, perhaps by increasing the trans effect of the bound NO; (ii) attack of the second NO on heme iron on the proximal side of the porphyrin with the subsequent rapid loss of both the histidine ligand and the attacking NO; and (iii) nucleophilic addition of NO to the bound NO generating a transient N-NO bond. Precedent exists in porphyrin chemistry for ii and iii (30–34). Except for early reports of sGC containing a bound copper ion (27), nothing is known about a second, non-heme NO binding site.
The β1-subunit heme domain forms homodimers and contains one heme per monomer (4). The spectroscopic properties are essentially identical to those of sGC (4, 35). This protein lacks the catalytic domain that is believed to be structurally adjusted after binding of NO to the heme and cleavage of the iron-histidine bond. Speculation on the activation of sGC has centered on the cleavage of the Fe-His bond and the subsequent flexing of the porphyrin plane as the triggers for conformational changes in the catalytic domain that activate sGC (26, 28, 36, 37). As described above, NO binds to β1(1–385) with direct conversion to the 5-coordinate ferrous-nitrosyl complex (Fig. 4A). The kon for binding of NO to β1(1–385) (7.1 ± 2 × 108 M−1⋅s−1) (Fig. 4B) is similar to that for heterodimeric sGC, indicating that this step was not greatly affected by the absence of the catalytic domain. If a 6-coordinate species is formed during the binding process (the process may actually be concerted), iron-histidine bond cleavage must occur fast enough to obscure any 6-coordinate intermediate. Therefore, iron-histidine bond cleavage in the fragment lacking the catalytic domain must be at least three orders of magnitude faster than in the heterodimeric enzyme, leading us to conclude that the energy required to drive catalysis in the heterodimer is not needed in the heme domain. Specifically, activation almost certainly involves a conformational change in the active-site brought about by NO binding to the heme. Moving those residues will require energy that is not needed when this domain is absent. Taken together, these findings then strongly suggest that the activation of sGC is triggered by the release of the axial histidine (His-β105) that is coordinated to the heme iron in the resting enzyme.
In summary, we have shown that sGC is an extremely efficient trap for NO, such that transport mechanisms are not a prerequisite for cell-to-cell signaling with NO. The rate of NO binding to sGC allows sGC to out-compete other reactions in the cell. The key step in the molecular process of activation of sGC is not binding of NO to heme; rather, it is the subsequent change in the heme coordination state. The rate of this activation step is regulated by the concentration of NO. This provides the basis for a sliding scale of signal transduction through this enzyme that permits, for example, maintenance of tone in vascular smooth muscle.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (M.A.M.), a Horace H. Rackham Predoctoral Fellowship (Y.Z.), and National Institutes of Health Grant GM-20877 (D.P.B.). This work was carried out during the tenure of a Research Fellowship (P.E.B.) of the American Heart Association, Michigan Affiliate.
Abbreviations
- sGC
soluble isoform of guanylate cyclase
- β1(1–385)
residues 1–385 of the β1 subunit
- NOS
nitric oxide synthase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Marletta M A. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro L J. Biochem Pharmacol. 1991;41:485–490. doi: 10.1016/0006-2952(91)90618-f. [DOI] [PubMed] [Google Scholar]
- 3.Denninger J W, Marletta M A. Biochim Biophys Acta. 1999;1411:334–350. doi: 10.1016/s0005-2728(99)00024-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Marletta M A. Biochemistry. 1997;36:15959–15964. doi: 10.1021/bi971825x. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Schevis J, Babcock G T, Marletta M A. Biochemistry. 1998;37:4502–4509. doi: 10.1021/bi972686m. [DOI] [PubMed] [Google Scholar]
- 6.Stone J R, Marletta M A. Biochemistry. 1996;35:1093–1099. doi: 10.1021/bi9519718. [DOI] [PubMed] [Google Scholar]
- 7.Ascenzi P, Coletta M, Desideri A, Petruzzelli R, Polizio F, Bolognesi M, Condo S G, Giardina B. J Inorg Biochem. 1992;45:31–37. doi: 10.1016/0162-0134(92)84038-o. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V J, Traylor T G, Gardiner R. Biochemistry. 1987;26:3837–3843. doi: 10.1021/bi00387a015. [DOI] [PubMed] [Google Scholar]
- 9.Moore E G, Gibson Q H. J Biol Chem. 1976;251:2788–2794. [PubMed] [Google Scholar]
- 10.Kelm M, Schrader J. Circ Res. 1990;66:1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- 11.Myers P R, Minor Jr R L, Guerra R, Jr, Bates J N, Harrison D G. Nature (London) 1990;345:161–163. doi: 10.1038/345161a0. [DOI] [PubMed] [Google Scholar]
- 12.Ignarro L J, Lippton H, Edwards J C, Baricos W H, Hyman A L, Kadowitz P J, Gruetter C A. J Pharmacol Exp Ther. 1981;218:739–749. [PubMed] [Google Scholar]
- 13.Singh S, Garbers D L. Methods Enzymol. 1991;195:414–423. doi: 10.1016/0076-6879(91)95188-p. [DOI] [PubMed] [Google Scholar]
- 14.Singh R J, Hogg N, Joseph J, Kalyanaraman B. J Biol Chem. 1996;271:18596–18603. doi: 10.1074/jbc.271.31.18596. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Rudd M A, Freedman J A, Loscalzo J. J Pharmacol Exp Ther. 1998;284:526–534. [PubMed] [Google Scholar]
- 16.Ignarro L J, Gruetter C A. Biochim Biophys Acta. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- 17.Mellion B T, Ignarro L J, Myers C B, Ohlstein E H, Ballot B A, Hyman A L, Kadowitz P J. Mol Pharmacol. 1983;23:653–664. [PubMed] [Google Scholar]
- 18.Muller B, Kleschyov A L, Stoclet J-C. Br J Pharmacol. 1996;119:1281–1285. doi: 10.1111/j.1476-5381.1996.tb16034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keese M A, Böse M, Mülsch A, Schirmer R H, Becker K. Biochem Pharmacol. 1997;54:1307–1313. doi: 10.1016/s0006-2952(97)00348-1. [DOI] [PubMed] [Google Scholar]
- 20.Stamler J S, Jia L, Eu J P, McMahon T J, Demchenko I T, Bonaventura J, Gernet K, Piantadosi C A. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 21.Gow A J, Stamler J S. Nature (London) 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 22.Brandish P E, Buechler W, Marletta M A. Biochemistry. 1998;37:16898–16907. doi: 10.1021/bi9814989. [DOI] [PubMed] [Google Scholar]
- 23.Bull C, Ballou D P. J Biol Chem. 1981;256:12673–12680. [PubMed] [Google Scholar]
- 24.Keefer L K, Nims R W, Davies K M, Wink D A. Methods Enzymol. 1996;268:281–293. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 25.Makino R, Matsuda H, Obayashi E, Shiro Y, Iizuka T, Hori H. J Biol Chem. 1999;274:7714–7723. doi: 10.1074/jbc.274.12.7714. [DOI] [PubMed] [Google Scholar]
- 26.Stone J R, Marletta M A. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 27.Gerzer R, Böhme E, Hofmann F, Schultz G. FEBS Lett. 1981;132:71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- 28.Ignarro L J, Wood K S, Wolin M S. Proc Natl Acad Sci USA. 1982;79:2870–2873. doi: 10.1073/pnas.79.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone J R, Sands R H, Dunham W R, Marletta M A. Biochem Biophys Res Commun. 1995;207:572–577. doi: 10.1006/bbrc.1995.1226. [DOI] [PubMed] [Google Scholar]
- 30.Wayland B B, Olson L W. J Am Chem Soc. 1974;96:6037–6041. doi: 10.1021/ja00826a013. [DOI] [PubMed] [Google Scholar]
- 31.Lançon D, Kadish K M. J Am Chem Soc. 1983;105:5610–5617. [Google Scholar]
- 32.Averill B A, Tiedje J M. FEBS Lett. 1982;138:8–12. doi: 10.1016/0014-5793(82)80383-9. [DOI] [PubMed] [Google Scholar]
- 33.Miranda K M, Bu X, Lorkovic I, Ford P C. Inorg Chem. 1997;36:4838–4848. doi: 10.1021/ic970065b. [DOI] [PubMed] [Google Scholar]
- 34.Addison A W, Stephanos J J. Biochemistry. 1986;25:4104–4113. doi: 10.1021/bi00362a018. [DOI] [PubMed] [Google Scholar]
- 35.Schelvis J P M, Zhao Y, Marletta M A, Babcock G T. Biochemistry. 1998;37:16289–16297. doi: 10.1021/bi981547h. [DOI] [PubMed] [Google Scholar]
- 36.Ignarro L J, Wood K S, Wolin M S. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:267–274. [PubMed] [Google Scholar]
- 37.Ignarro L J, Adams J B, Horwitz P M, Wood K S. J Biol Chem. 1986;261:4997–5002. [PubMed] [Google Scholar]
- 38.Kharitonov V G, Sharma V S, Magde D, Koesling D. Biochemistry. 1997;36:6814–6818. doi: 10.1021/bi970201o. [DOI] [PubMed] [Google Scholar]
- 39.Morris R J, Gibson Q H. J Biol Chem. 1980;255:8050–8053. [PubMed] [Google Scholar]
- 40.Doyle M P, Hoekstra J W. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]