Abstract
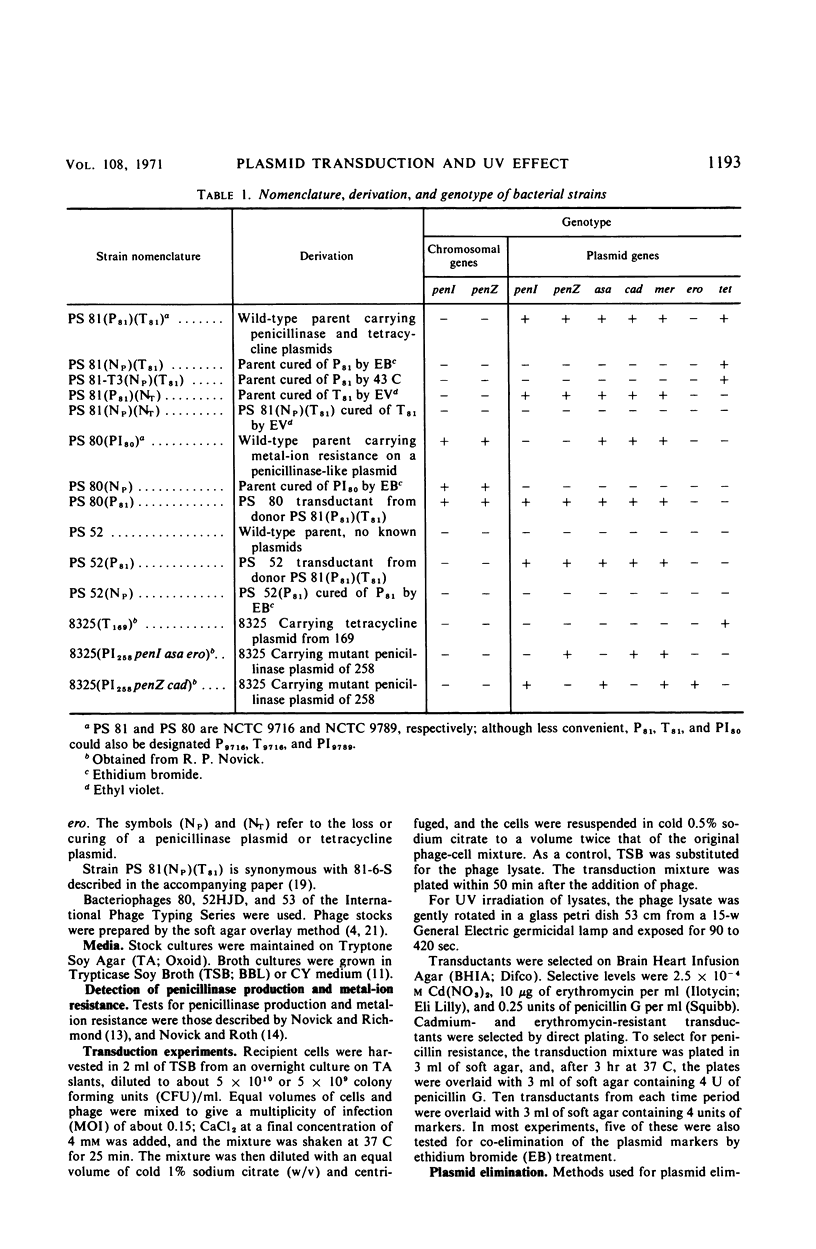
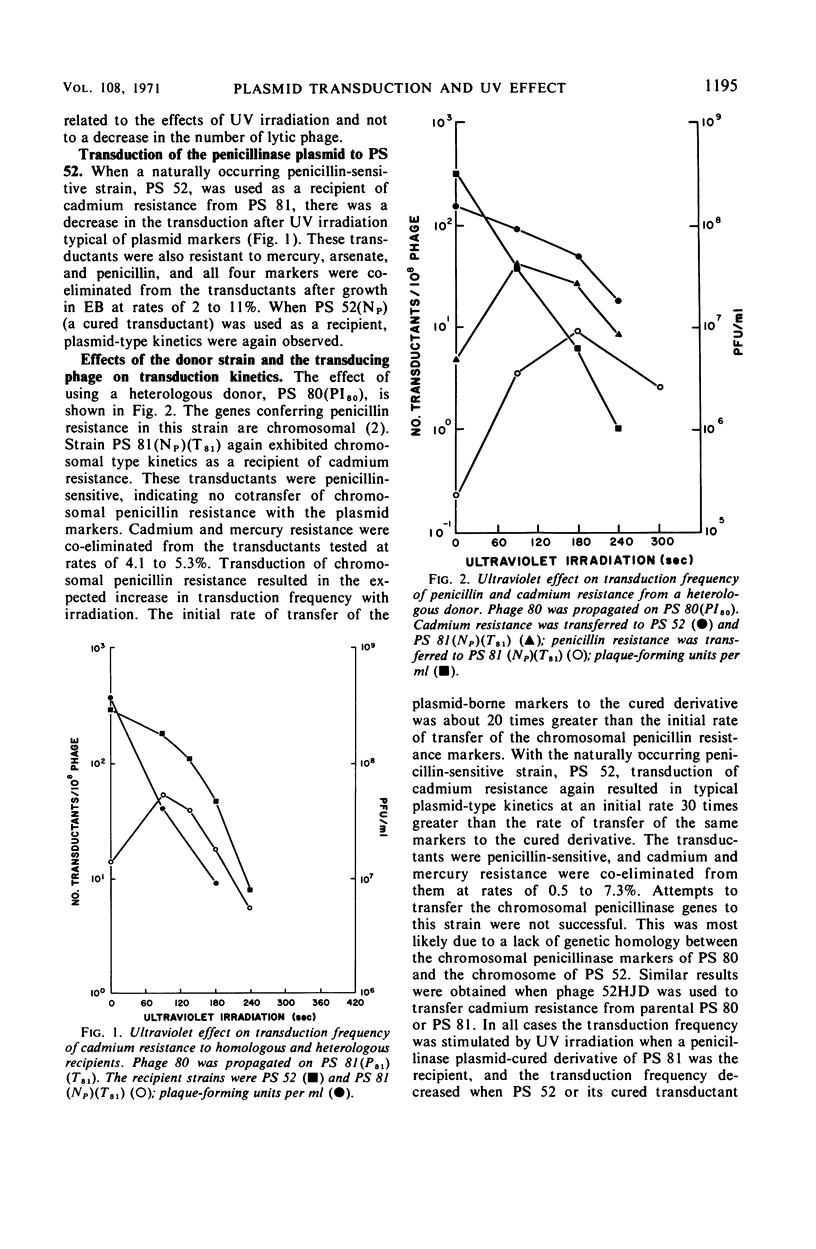
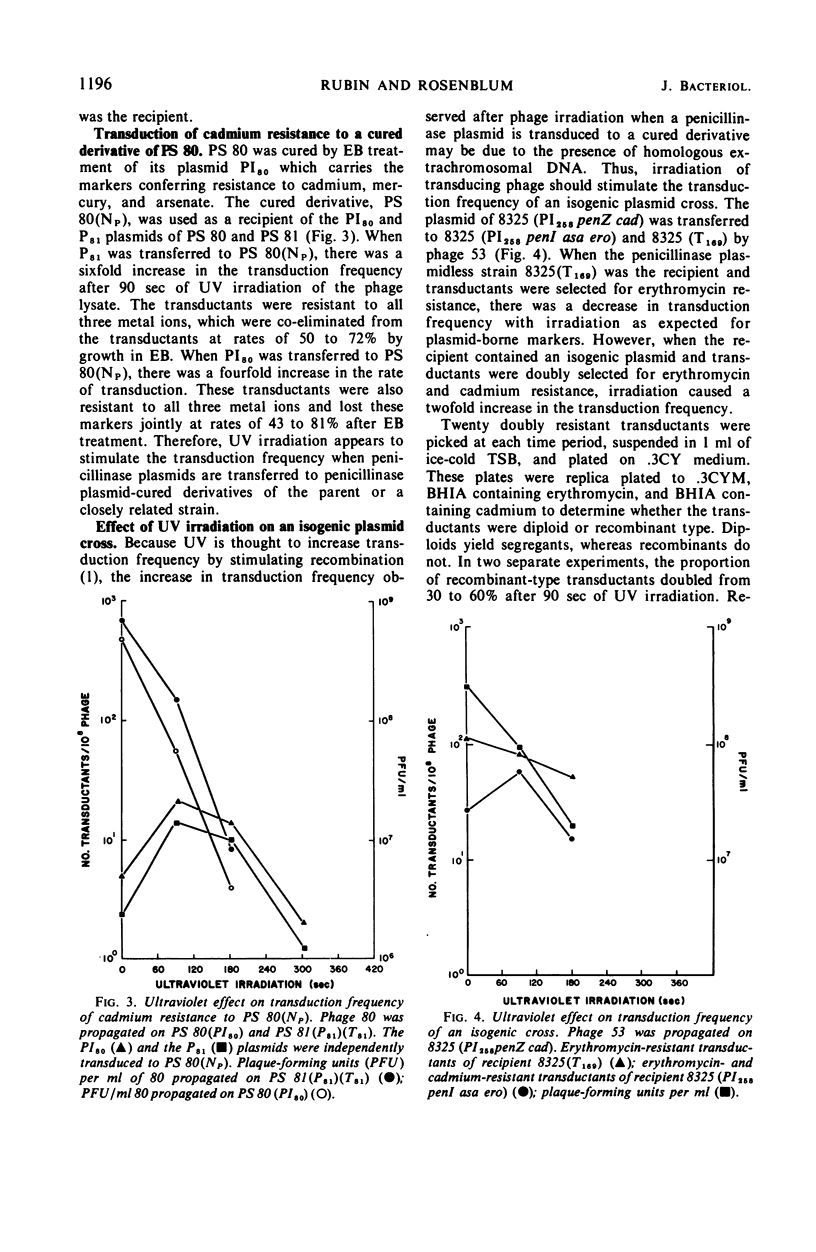
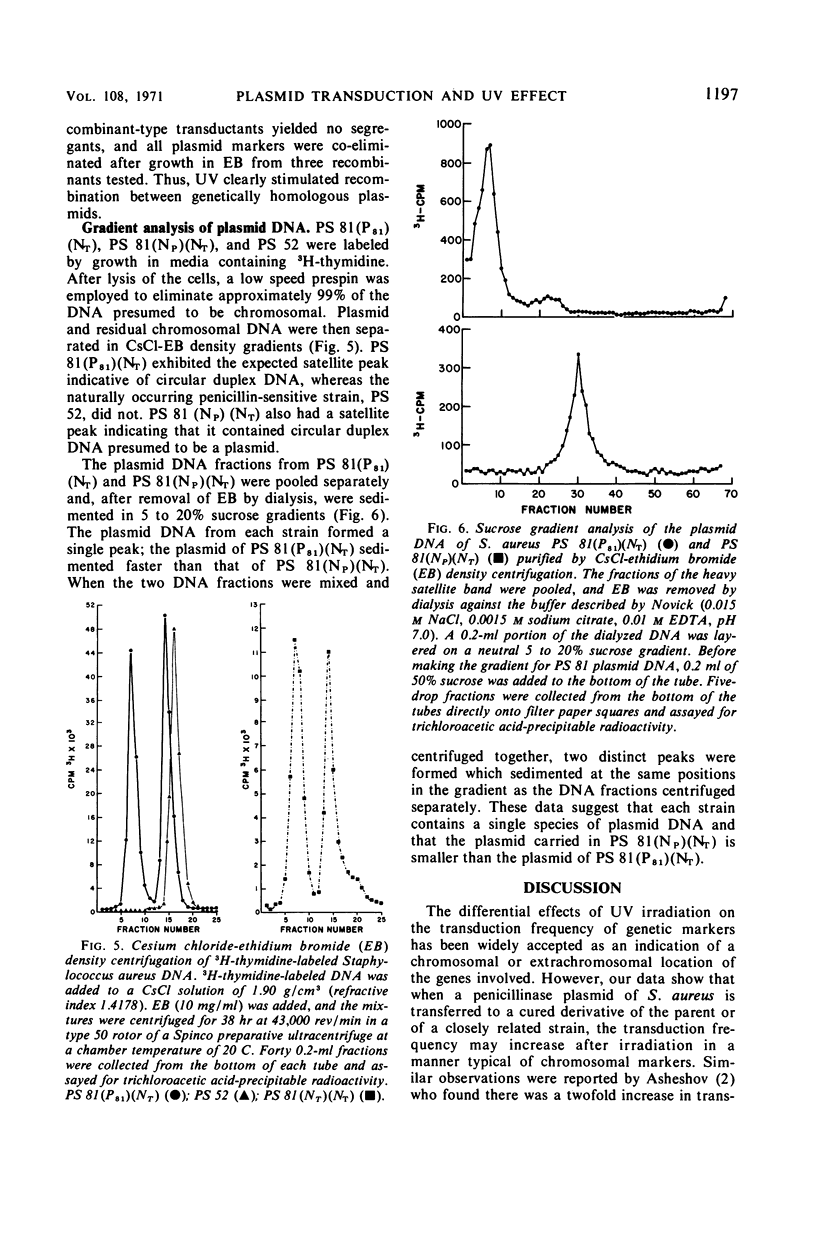
When the penicillinase plasmid of Staphylococcus aureus PS 81(P81)(T81) was transferred to its cured derivative of PS 81(NP)(T81), there was a fivefold increase in the transduction frequency of penicillinase plasmid markers after ultraviolet (UV) irradiation of the phage instead of the expected decrease typical for plasmid-borne markers. These results were independent of the transducing phage, the donor, and the method of curing the recipient and were also obtained with a cured derivative of PS 80(PI80). With PS 52, a naturally occurring penicillin-sensitive strain, and a cured transductant of PS 52 as the recipients, typical plasmid kinetics were observed. The plasmid location of penicillinase plasmid markers in transductants was confirmed by their instability in ethidium bromide (EB). In a cross between isogenic plasmids (PI258penZ cad × PI258penI asa ero), transductants were doubly selected for cadmium and erythromycin resistances. There was a twofold increase in transduction frequency after UV irradiation of the transducing phage and an increase in the proportion of recombinant type transductants. CsCl-EB density centrifugation revealed that plasmid deoxyribonucleic acid (DNA) was present in PS 81(P81)(NT) and its cured derivative [PS 81(NP)(NT)], but not in PS 52. Sucrose gradient analysis of plasmid DNA showed that the penicillinase plasmid of PS 81(P81)(NT) was larger than the plasmid in its cured derivative. Thus, the cured derivative contains plasmid DNA which appears to recombine with the incoming plasmid, causing the rise in transduction frequency noted after UV irradiation of transducing phage.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W. Transduction of chromosomal genes and episomes in Escherichia coli. Virology. 1960 May;11:273–288. doi: 10.1016/0042-6822(60)90066-0. [DOI] [PubMed] [Google Scholar]
- Asheshov E. H. Chromosomal location of the genetic elements controlling penicillinase production in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):804–806. doi: 10.1038/210804a0. [DOI] [PubMed] [Google Scholar]
- BLAIR J. E., CARR M. The bacteriophage typing of staphylococci. J Infect Dis. 1953 Jul-Aug;93(1):1–13. doi: 10.1093/infdis/93.1.1. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G., Parker M. T., Richmond M. H. Penicillinase production and metal-ion resistance in Staphylococcus aureus cultures isolated from hospital patients. J Med Microbiol. 1970 Feb;3(1):125–136. doi: 10.1099/00222615-3-1-125. [DOI] [PubMed] [Google Scholar]
- KORMAN R. Z., BERMAN D. T. Genetic transduction with staphylophage. J Bacteriol. 1962 Aug;84:228–236. doi: 10.1128/jb.84.2.228-236.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasatiya S. S., Baldwin J. N. Nature of the determinant of tetracycline resistance in Staphylococcus aureus. Can J Microbiol. 1967 Aug;13(8):1079–1086. doi: 10.1139/m67-144. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyru G., Wexler L. F., Novick R. P. Naturally occurring penicillinase plasmids in Staphylococcus aureus. J Bacteriol. 1969 Apr;98(1):215–221. doi: 10.1128/jb.98.1.215-221.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston S. M. Cellular location of the genes controlling penicillinase production and resistance to streptomycin and tetracycline in a strain of Staphylococcus aureus. Nature. 1966 May 21;210(5038):802–804. doi: 10.1038/210802a0. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Johnston J. The reversible transition of certain genes in Staphylococcus aureus between the integrated and the extra-chromosomal state. Genet Res. 1969 Jun;13(3):267–274. doi: 10.1017/s0016672300002950. [DOI] [PubMed] [Google Scholar]
- Rubin S. J., Rosenblum E. D. Effects of ethidium bromide on growth and on loss of the penicillinase plasmid of Staphylococcus aureus. J Bacteriol. 1971 Dec;108(3):1200–1204. doi: 10.1128/jb.108.3.1200-1204.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush M. G., Gordon C. N., Novick R. P., Warner R. C. Penicillinase plasmid DNA from Staphylococcus aureus. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1304–1310. doi: 10.1073/pnas.63.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]



