Abstract
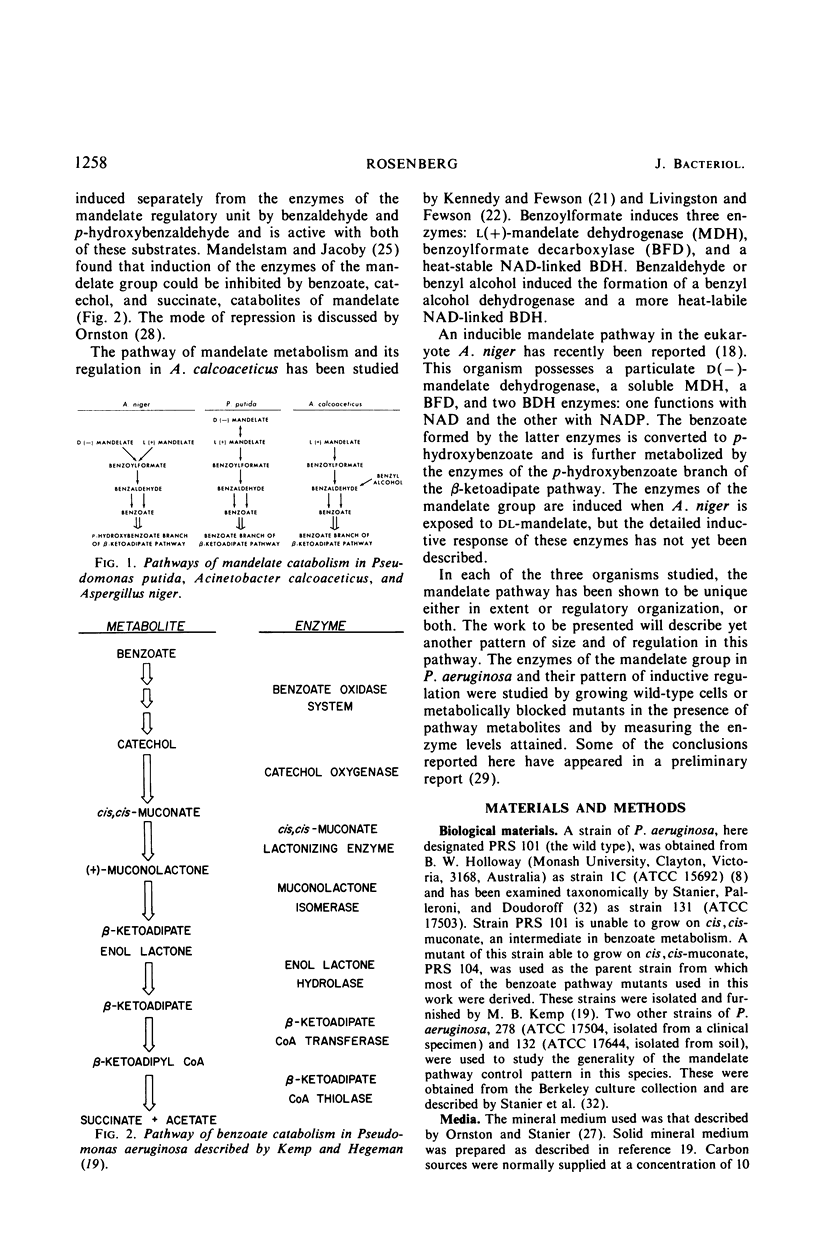
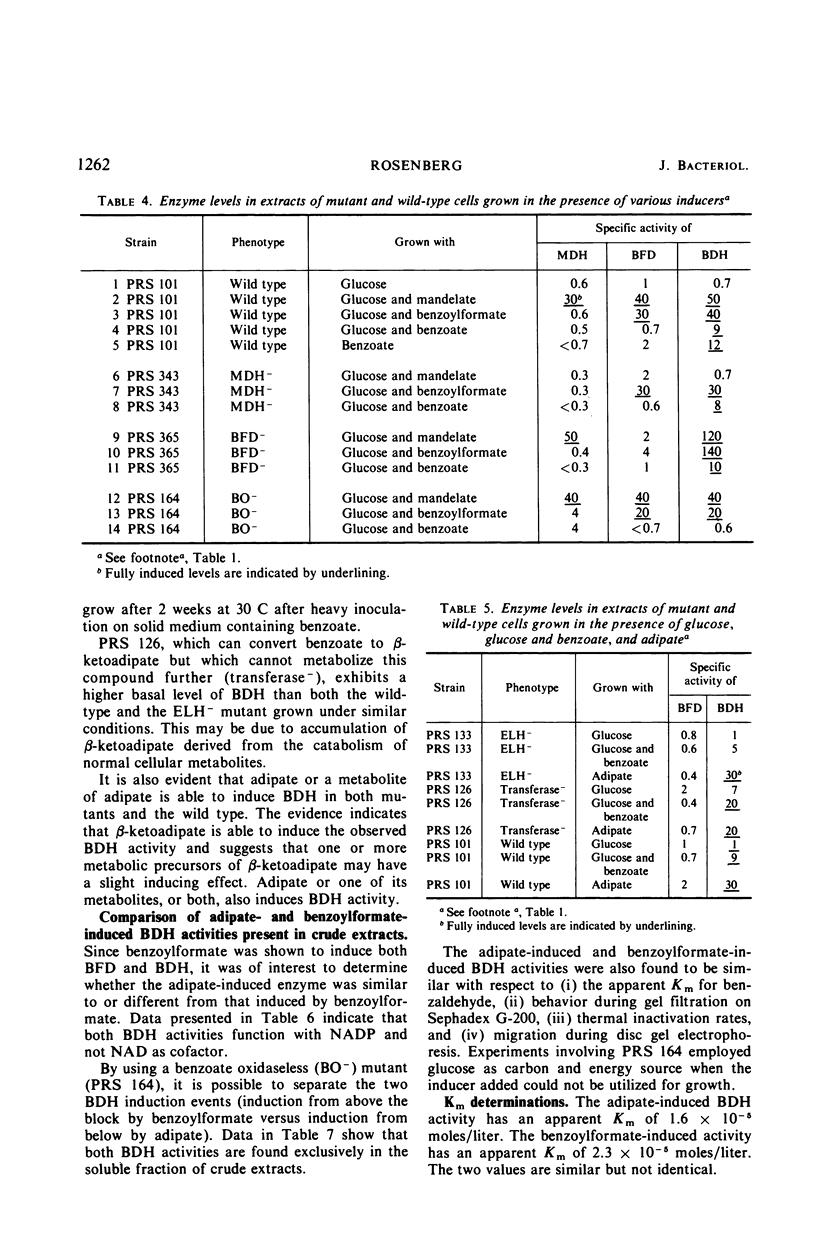
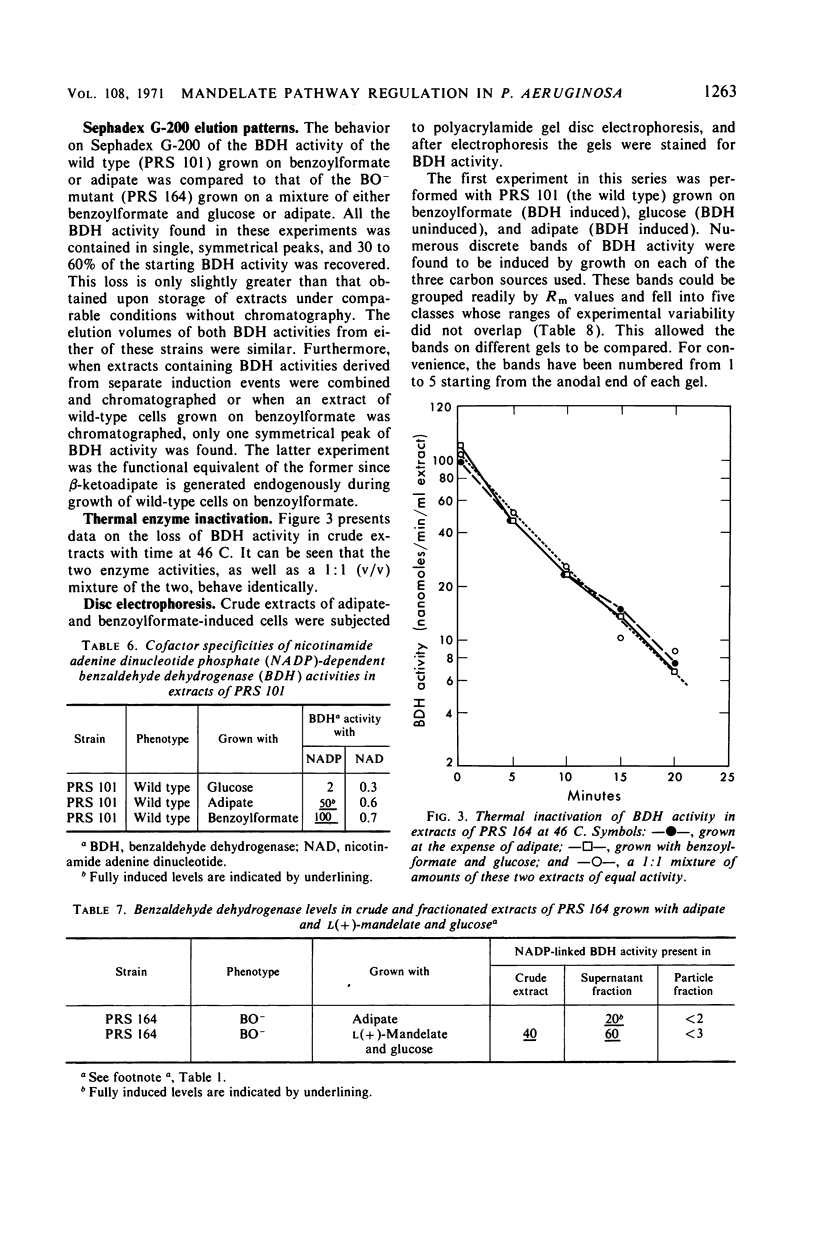
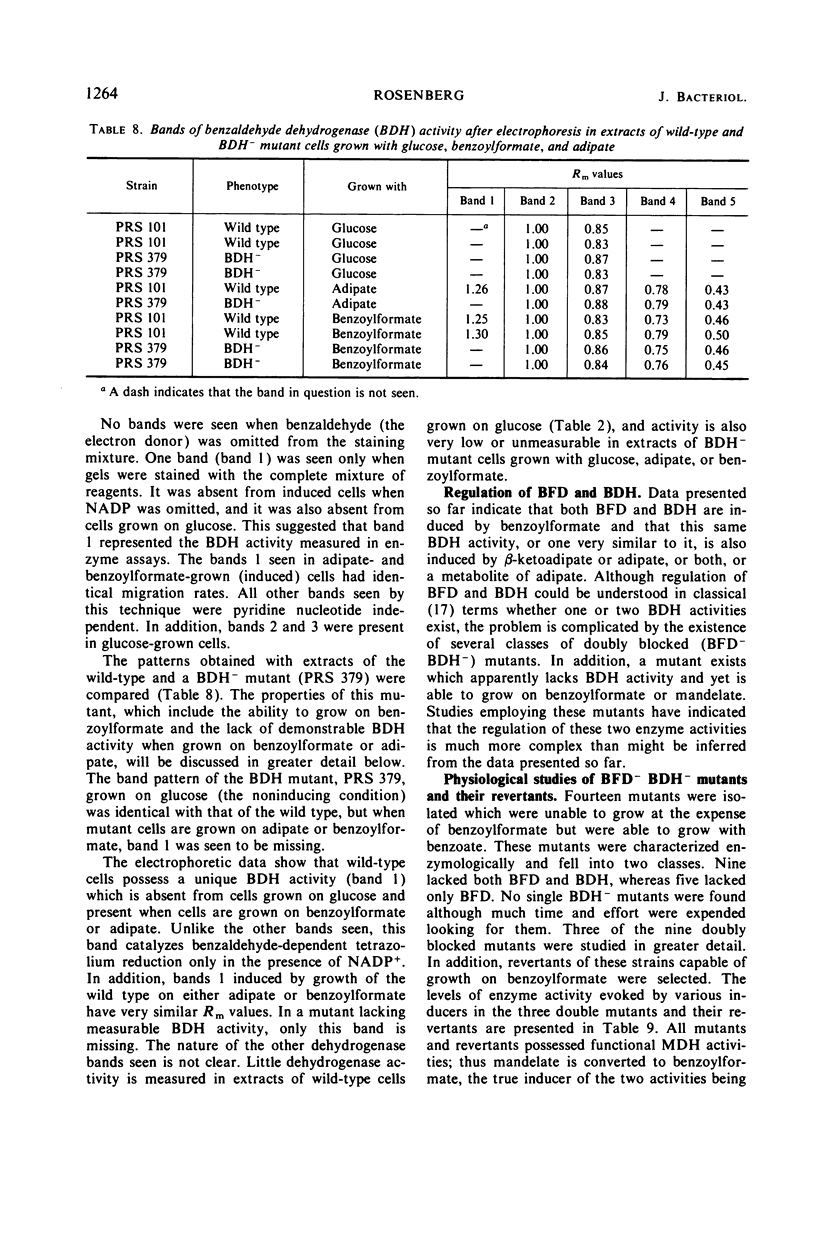
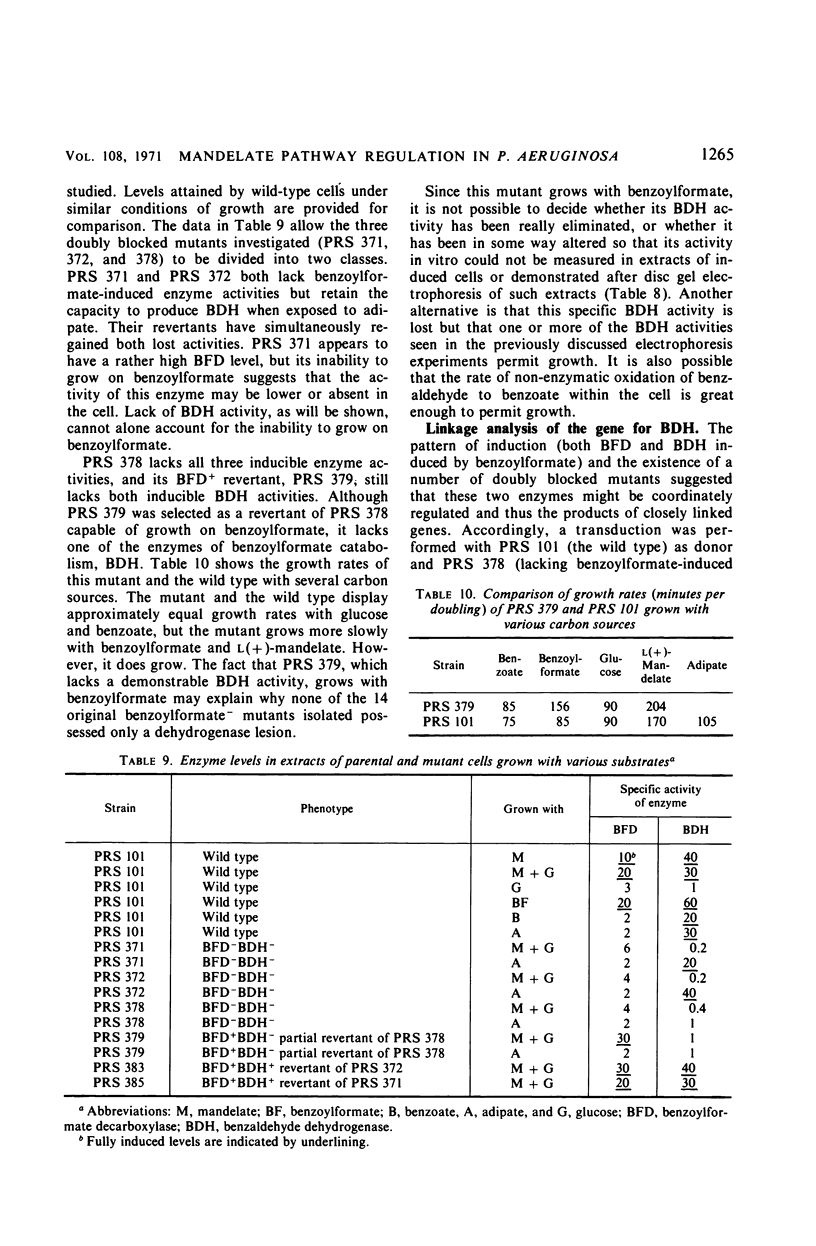
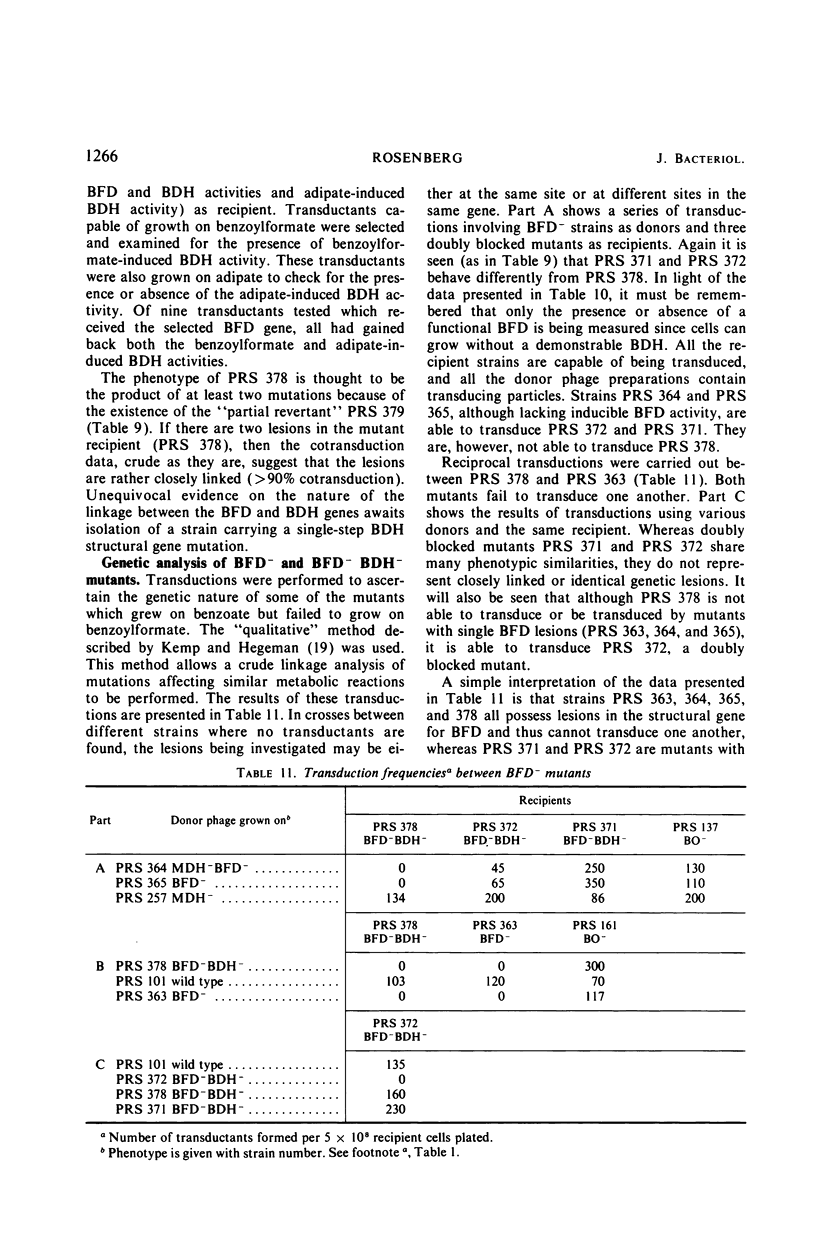
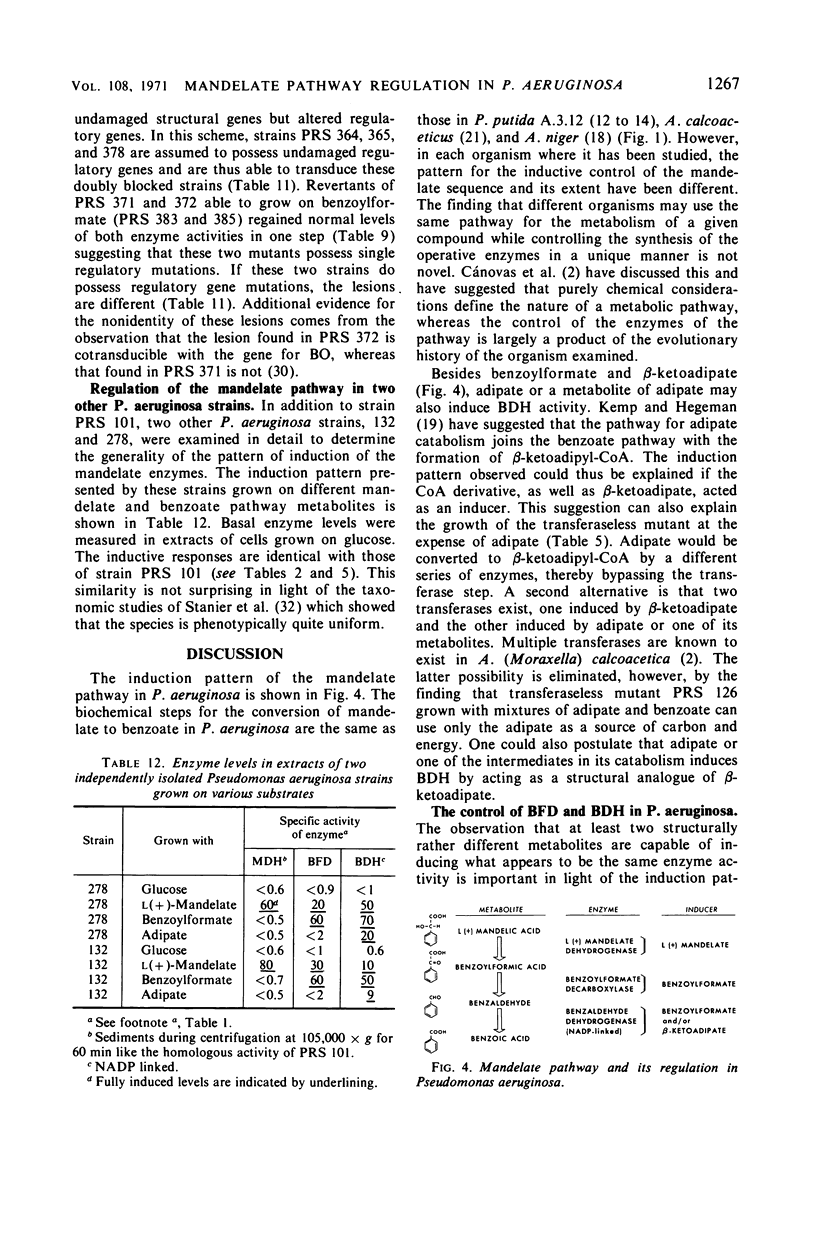
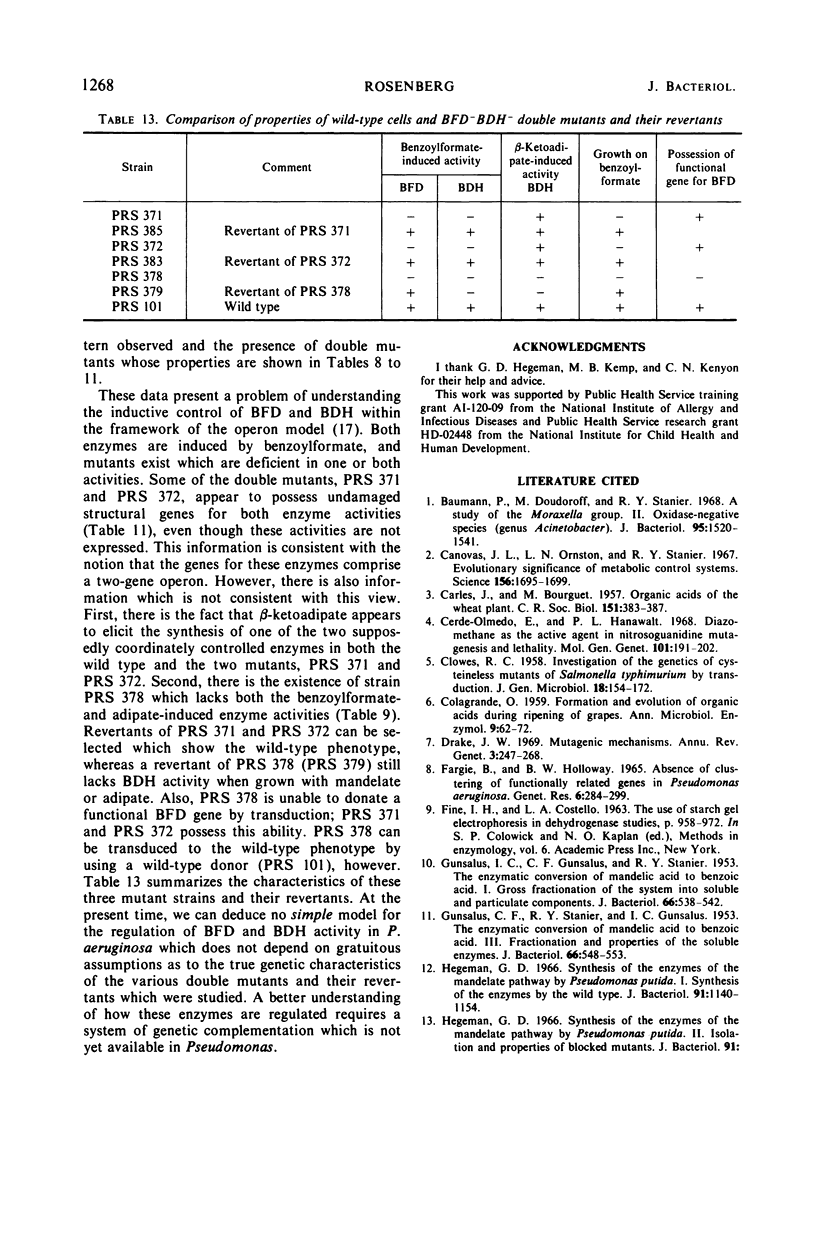
The pathway of mandelate metabolism in Pseudomonas aeruginosa is composed of the following steps: l(+)-mandelate → benzoylformate → benzaldehyde → benzoate. These three steps are unique to mandelate oxidation; the benzoate formed is further metabolized via the β-ketoadipate pathway. The first enzyme, l(+)-mandelate dehydrogenase, is induced by its substrate. The second and third enzymes, benzoylformate decarboxylase and benzaldehyde dehydrogenase, are both induced by benzoylformate. The same benzaldehyde dehydrogenase, or one very similar to it, is also induced by β-ketoadipate, an intermediate in the subsequent metabolism of benzoate. This dehydrogenase may also be induced by adipate or a metabolite of adipate. These conclusions have been drawn from the physiological and genetic properties of wild-type P. aeruginosa strains and from the study of mutants lacking the second and third enzyme activities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON J. F., CAIN B. F. Antileukaemic activity of polyporic acid. Nature. 1959 Oct 24;184(Suppl 17):1326–1327. doi: 10.1038/1841326a0. [DOI] [PubMed] [Google Scholar]
- Baumann P., Doudoroff M., Stanier R. Y. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol. 1968 May;95(5):1520–1541. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLES J., BOURGUET M. Les acides organiques du blé. C R Seances Soc Biol Fil. 1957;151(2):383–387. [PubMed] [Google Scholar]
- CLOWES R. C. Investigation of the genetics of cysteineless mutants of Salmonella typhimurium by transduction. J Gen Microbiol. 1958 Feb;18(1):154–172. doi: 10.1099/00221287-18-1-154. [DOI] [PubMed] [Google Scholar]
- Cerdá-Olmedo E., Hanawalt P. C. Diazomethane as the active agent in nitrosoguanidine mutagenesis and lethality. Mol Gen Genet. 1968 May 3;101(3):191–202. doi: 10.1007/BF00271621. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- FARGIE B., HOLLOWAY B. W. ABSENCE OF CLUSTERING OF FUNCTIONALLY RELATED GENES IN PSEUDOMONAS AERUGINOSA. Genet Res. 1965 Jul;6:284–299. doi: 10.1017/s0016672300004158. [DOI] [PubMed] [Google Scholar]
- GUNSALUS C. F., STANIER R. Y., GUNSALUS I. C. The enzymatic conversion of mandelic acid to benzoic acid. III. Fractionation and properties of the soluble enzymes. J Bacteriol. 1953 Nov;66(5):548–553. doi: 10.1128/jb.66.5.548-553.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNSALUS I. C., GUNSALUS C. F., STANIER R. Y. The enzymatic conversion of mandelic acid to benzoic acid. I. Gross fractionation of the system into soluble and particulate components. J Bacteriol. 1953 Nov;66(5):538–542. doi: 10.1128/jb.66.5.538-542.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg E. Y., Kenyon G. L. Mandelic acid racemase from Pseudomonas putida. Purification and properties of the enzyme. Biochemistry. 1970 Oct 13;9(21):4029–4036. doi: 10.1021/bi00823a001. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. 3. Isolation and properties of constitutive mutants. J Bacteriol. 1966 Mar;91(3):1161–1167. doi: 10.1128/jb.91.3.1161-1167.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Jamaluddin M., Rao P. V., Vaidyanathan C. S. Involvement of the protocatechuate pathway in the metabolism of mandelic acid by Aspergillus niger. J Bacteriol. 1970 Mar;101(3):786–793. doi: 10.1128/jb.101.3.786-793.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. B., Hegeman G. D. Genetic control of the beta-ketoadipate pathway in Pseudomonas aeruginosa. J Bacteriol. 1968 Nov;96(5):1488–1499. doi: 10.1128/jb.96.5.1488-1499.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Enzymes of the mandelate pathway in Bacterium N.C.I.B. 8250. Biochem J. 1968 Apr;107(4):497–506. doi: 10.1042/bj1070497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. I., Fewson C. A. Metabolism of mandelate and related compounds by bacterium NCIB 8250. J Gen Microbiol. 1968 Sep;53(2):259–273. doi: 10.1099/00221287-53-2-259. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Livingstone A., Fewson C. A. Regulation of enzymes converting L-mandelate into benzoate in bacterium N.C.I.B. 8250. Biochem J. 1971 Jan;121(1):8P–9P. doi: 10.1042/bj1210008pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K., MCFALL E. GENETIC ASPECTS OF METABOLIC CONTROL. Annu Rev Microbiol. 1964;18:95–110. doi: 10.1146/annurev.mi.18.100164.000523. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. Regulation of catabolic pathways in Pseudomonas. Bacteriol Rev. 1971 Jun;35(2):87–116. doi: 10.1128/br.35.2.87-116.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Rosenberg S. L., Hegeman G. D. Clustering of functionally related genes in Pseudomonas aeruginosa. J Bacteriol. 1969 Jul;99(1):353–355. doi: 10.1128/jb.99.1.353-355.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., GUNSALUS I. C., GUNSALUS C. F. The enzymatic conversion of mandelic acid to benzoic acid. II. Properties of the particulate fractions. J Bacteriol. 1953 Nov;66(5):543–547. doi: 10.1128/jb.66.5.543-547.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L., Mandelstam J. Induction and multi-sensitive end-product repression in two converging pathways degrading aromatic substances in Pseudomonas fluorescens. Biochem J. 1965 Aug;96(2):354–362. doi: 10.1042/bj0960354. [DOI] [PMC free article] [PubMed] [Google Scholar]



