Abstract
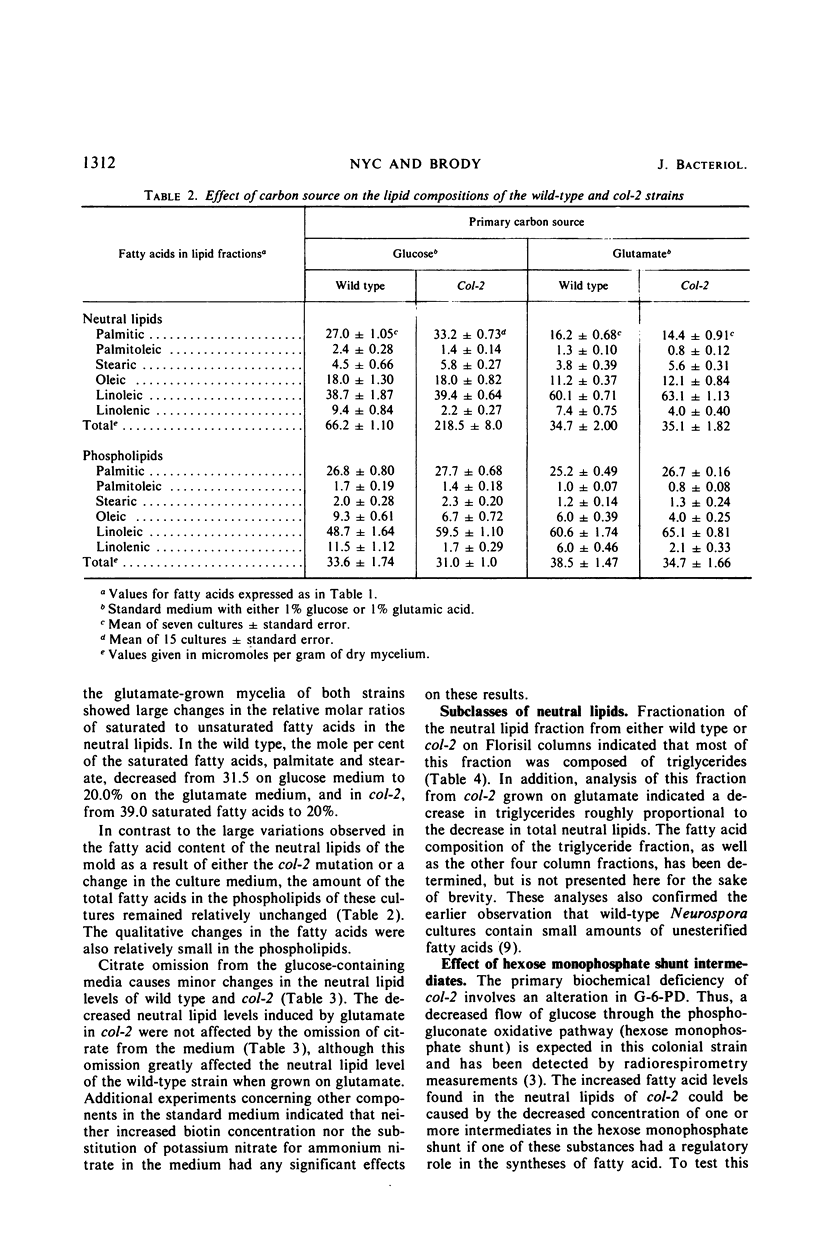
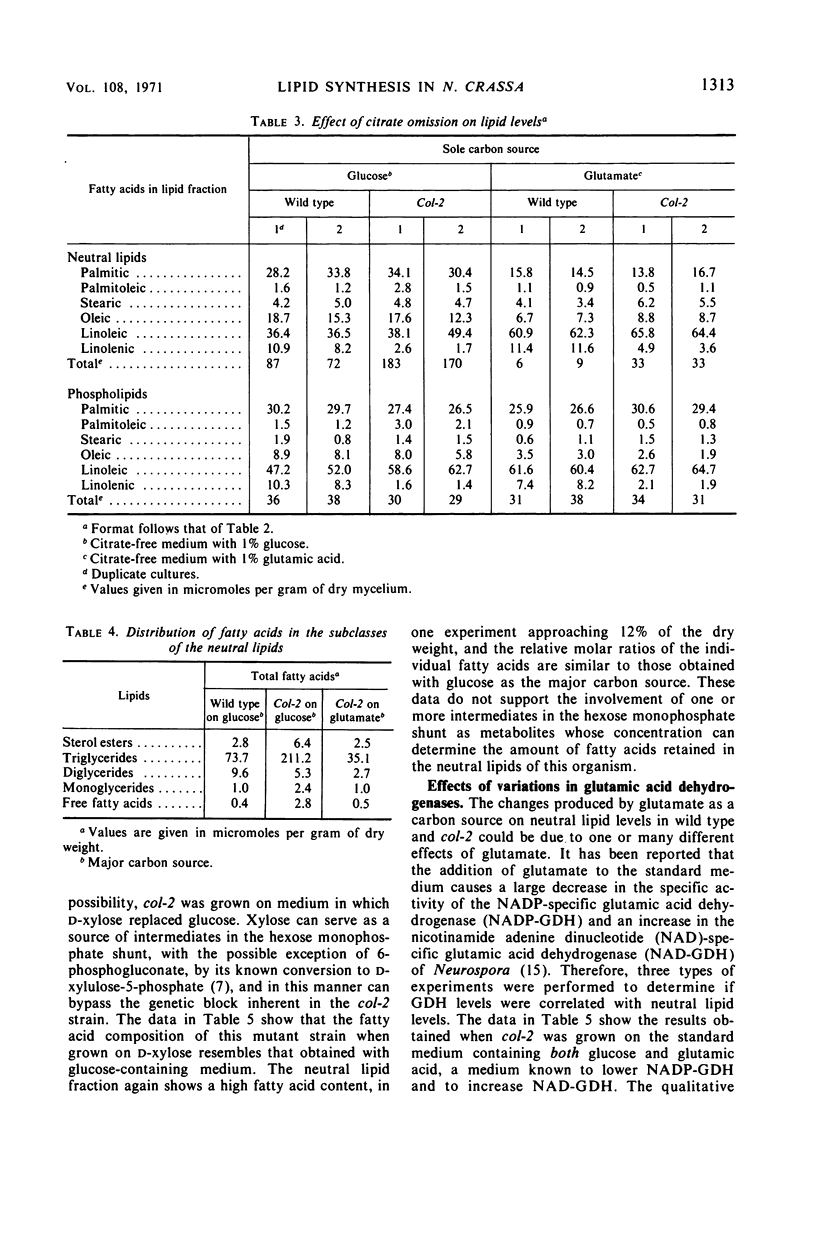
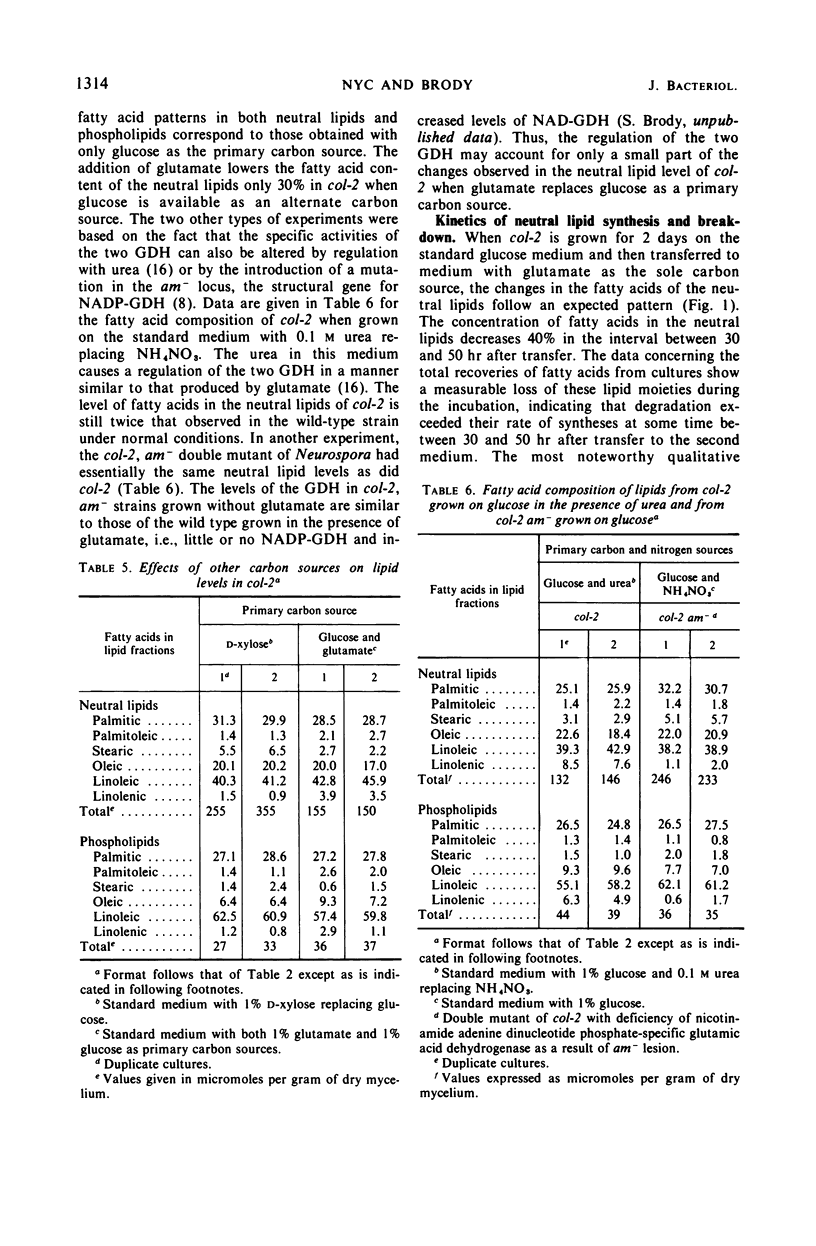
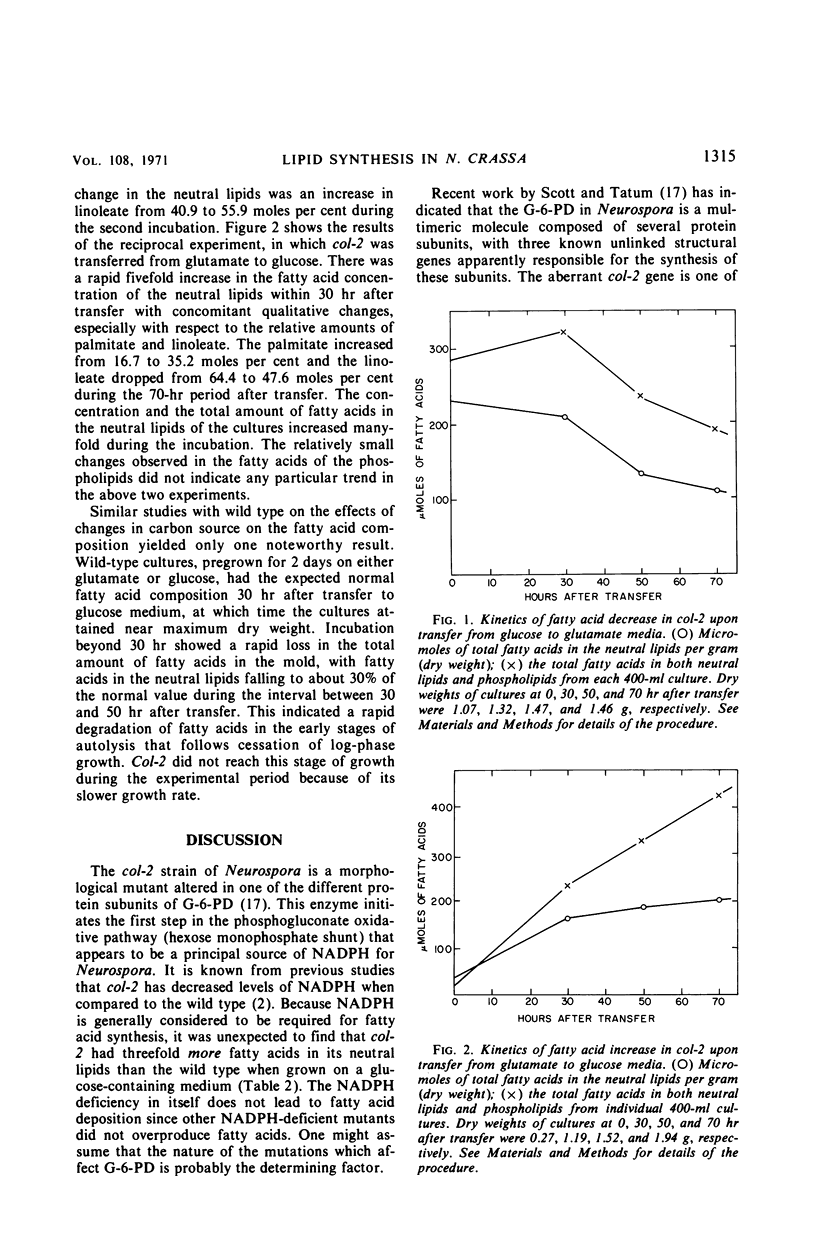
A morphological mutant (col-2) of Neurospora, which is partially deficient in glucose-6-phosphate dehydrogenase (G-6-PD) activity and has lower levels of reduced nicotinamide adenine dinucleotide phosphate (NADPH), accumulated three-fold more triglycerides during log-phase growth than the wild-type strain. Increased lipid deposition was not found in other strains that included slow-growing morphological mutants, NADPH-deficient strains, G-6-PD-deficient mutants, wild-type revertants from col-2, and a cel, col-2 double mutant. The cel, col-2 strain was supplemented with an exogenous source of fatty acids because it cannot synthesize these lipid moieties. The observed normal lipid content of this strain suggests that the lipid deposition in col-2 on glucose is due to an overstimulation of fatty acid synthesis and not a deficiency in fatty acid breakdown. The neutral lipid levels in both wild type and col-2 were decreased to identical levels when grown on glutamate as a carbon source. This effect was not due to changes in glutamic dehydrogenase levels. The omission of citrate from the glutamate medium reduced wild-type neutral lipid levels even further, but had no effect on col-2. The variations with time in the neutral lipid levels of col-2 upon changes in these carbon sources are presented, as well as a discussion of the possible types of regulatory effects unique to the col-2 mutation which might affect fatty acid synthesis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal P. K., Canvin D. T. The pentose phosphate pathway in relation to fat synthesis in the developing castor oil seed. Plant Physiol. 1971 May;47(5):672–675. doi: 10.1104/pp.47.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. Correlation between reduced nicotinamide adenine dinucleotide phosphate levels and morphological changes in Neurospora crassa. J Bacteriol. 1970 Mar;101(3):802–807. doi: 10.1128/jb.101.3.802-807.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S., Nyc J. F. Altered fatty acid distribution in mutants of Neurospora crassa. J Bacteriol. 1970 Nov;104(2):780–786. doi: 10.1128/jb.104.2.780-786.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S., Tatum E. L. The primary biochemical effect of a morphological mutation in Neurospora crassa. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1290–1297. doi: 10.1073/pnas.56.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARROLL K. K. Separation of lipid classes by chromatography on Florisil. J Lipid Res. 1961 Apr;2:135–141. [PubMed] [Google Scholar]
- CHIANG C., KNIGHT S. G. Metabolism of d-xylose by moulds. Nature. 1960 Oct 1;188:79–81. doi: 10.1038/188079a0. [DOI] [PubMed] [Google Scholar]
- FINCHAM J. R. A modified glutamic acid dehydrogenase as a result of gene mutation in Neurospora crassa. Biochem J. 1957 Apr;65(4):721–728. doi: 10.1042/bj0650721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDESTY B. A., MITCHELL H. K. The accumulation of free fatty acids in poky, a maternally inherited mutant of Neurospora crassa. Arch Biochem Biophys. 1963 Feb;100:330–334. doi: 10.1016/0003-9861(63)90081-x. [DOI] [PubMed] [Google Scholar]
- Henry S. A., Keith A. D. Saturated fatty acid requirer of Neurospora crassa. J Bacteriol. 1971 Apr;106(1):174–182. doi: 10.1128/jb.106.1.174-182.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelovich L., McGrath H. Pathways of fatty acid biosynthesis: effect of glucose and insulin on the conversion of glutamate carbon to fatty acid carbon via citrate by prelactating tissues and hyperplastic alveolar nodule outgrowths. Biochim Biophys Acta. 1970 Oct 6;218(1):18–28. doi: 10.1016/0005-2760(70)90088-3. [DOI] [PubMed] [Google Scholar]
- LEIN J., LEIN P. S. The production of acetate from fatty acids by Neurospora. J Bacteriol. 1950 Aug;60(2):185–190. doi: 10.1128/jb.60.2.185-190.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M. D., Edwards J., Stoll E., Moss J. Tricarboxylic acid activator-induced changes at the active site of acetyl-CoA carboxylase. Vitam Horm. 1970;28:345–363. doi: 10.1016/s0083-6729(08)60902-4. [DOI] [PubMed] [Google Scholar]
- Numa S., Nakanishi S., Hashimoto T., Iritani N., Okazaki T. Role of acetyl coenzyme A carboxylase in the control of fatty acid synthesis. Vitam Horm. 1970;28:213–243. doi: 10.1016/s0083-6729(08)60895-x. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Concurrent regulation of glutamic acid dehydrogenases of Neurospora. Arch Biochem Biophys. 1962 Jun;97:582–588. doi: 10.1016/0003-9861(62)90127-3. [DOI] [PubMed] [Google Scholar]
- SANWAL B. D., LATA M. Effect of glutamic acid on the formation of two glutamic acid dehydrogenases of Neurospora. Biochem Biophys Res Commun. 1962 Jan 24;6:404–409. doi: 10.1016/0006-291x(62)90364-9. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Tatum E. L. Glucose-6-phosphate dehydrogenase and Neurospora morphology. Proc Natl Acad Sci U S A. 1970 Jun;66(2):515–522. doi: 10.1073/pnas.66.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]



