Abstract
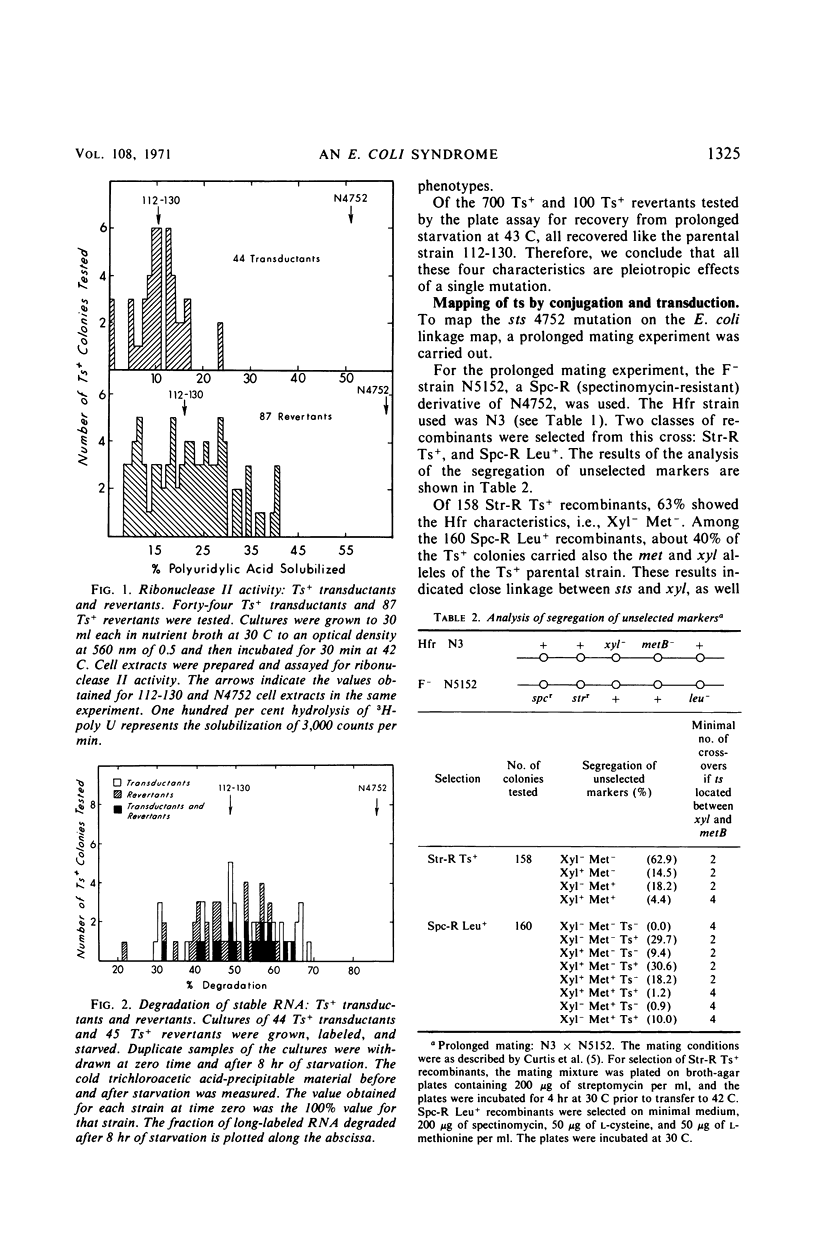
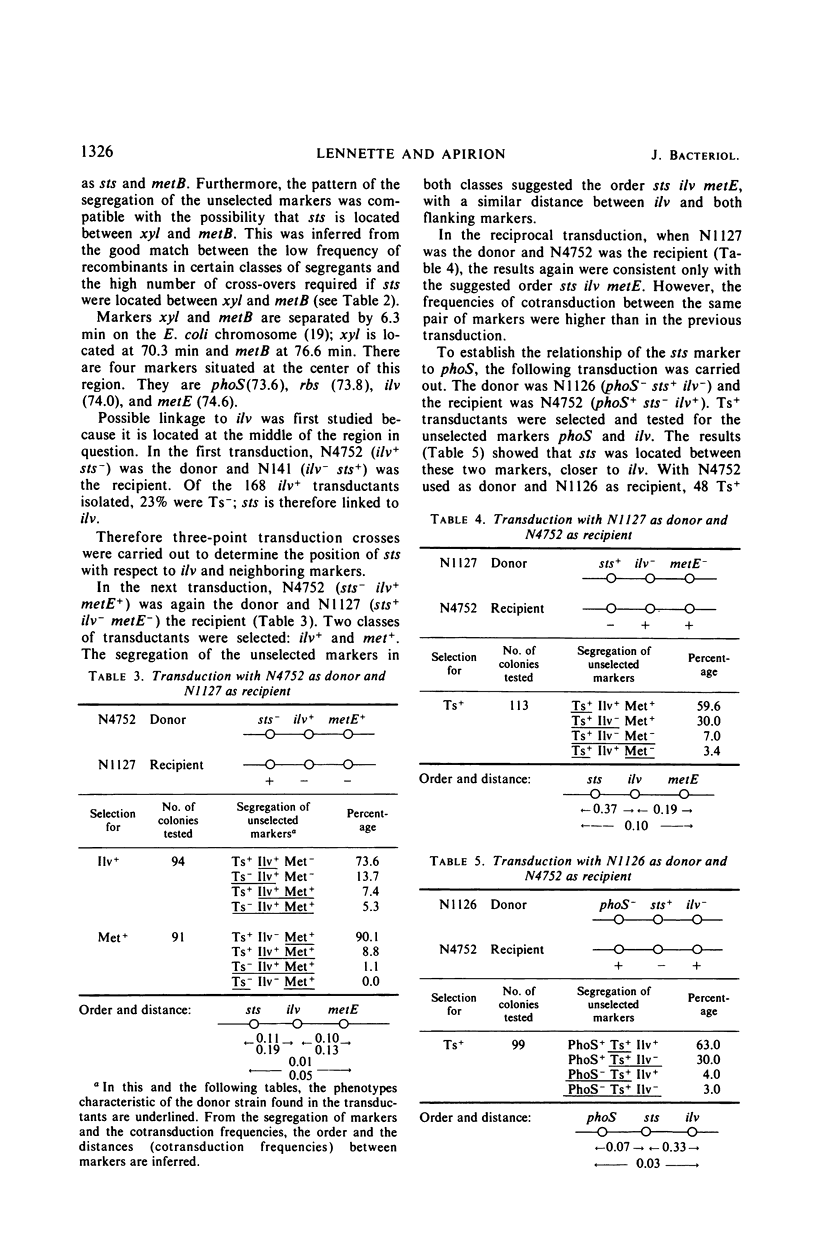
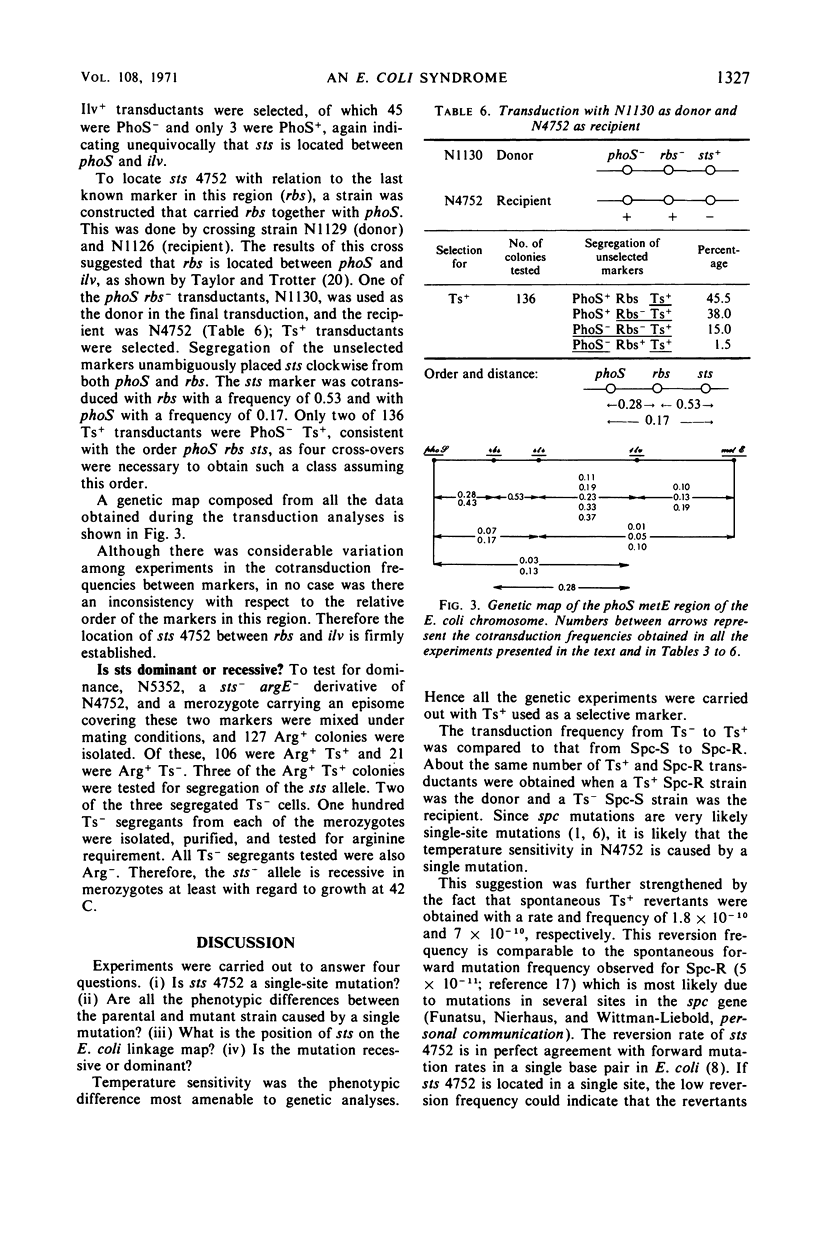
A mutant strain of Escherichia coli that fails to recover from prolonged (72 hr) starvation also fails to grow at 43 C. Extracts of this mutant strain show an increased ribonuclease II activity as compared to extracts of the parental strain, and stable ribonucleic acid is degraded to a larger extent in this strain during starvation. Ts+ transductants and revertants were tested for all the above-mentioned phenotypes. All the Ts+ transductants and revertants tested behaved like the Ts+ parental strain, which suggests that all the observed phenotypes are caused by a single sts (starvation-temperature sensitivity) mutation. The reversion rate from sts− to sts+ is rather low but is within the range of reversion rates for other single-site mutations. Three-point transduction crosses located this sts mutation between the ilv and rbs genes. The properties of sts+/sts− merozygotes suggested that the Ts− phenotype of this mutation is recessive.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. Sensitivity and Resistance to Spectinomycin in Escherichia coli. J Bacteriol. 1969 Nov;100(2):939–947. doi: 10.1128/jb.100.2.939-947.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D. Altered ribosomes in a suppressor strain of Escherichia coli. J Mol Biol. 1966 Apr;16(2):285–301. doi: 10.1016/s0022-2836(66)80173-0. [DOI] [PubMed] [Google Scholar]
- Apirion D., Phillips S. L., Schlessinger D. Approaches to the genetics of Escherichia coli ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:117–128. doi: 10.1101/sqb.1969.034.01.018. [DOI] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Coresistance to neomycin and kanamycin by mutations in an Escherichia coli locus that affects ribosomes. J Bacteriol. 1968 Sep;96(3):768–776. doi: 10.1128/jb.96.3.768-776.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Stallions D. R., Mays J. A. Parental functions during conjugation in Escherichia coli K-12. Bacteriol Rev. 1968 Dec;32(4 Pt 1):320–348. [PMC free article] [PubMed] [Google Scholar]
- Davies J., Anderson P., Davis B. D. Inhibition of protein synthesis by spectinomycin. Science. 1965 Sep 3;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. Comparative rates of spontaneous mutation. Nature. 1969 Mar 22;221(5186):1132–1132. doi: 10.1038/2211132a0. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazzarini R. A., Dahlberg A. E. The control of ribonucleic acid synthesis during amino acid deprivation in Escherichia coli. J Biol Chem. 1971 Jan 25;246(2):420–429. [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson B. M., Strigini P. F., Beckwith J. R. Allelic amber and ochre suppressors. J Mol Biol. 1968 Sep 14;36(2):209–218. doi: 10.1016/0022-2836(68)90376-8. [DOI] [PubMed] [Google Scholar]
- Phillips S. L., Schlessinger D., Apirion D. Mutants in Escherichia coli ribosomes: a new selection. Proc Natl Acad Sci U S A. 1969 Mar;62(3):772–777. doi: 10.1073/pnas.62.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. L., Schlessinger D., Apirion D. Temperature-dependent suppression of UGA and UAA codons in a temperature-sensitive mutant of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1969;34:499–503. doi: 10.1101/sqb.1969.034.01.056. [DOI] [PubMed] [Google Scholar]
- Pittard J., Wallace B. J. Distribution and function of genes concerned with aromatic biosynthesis in Escherichia coli. J Bacteriol. 1966 Apr;91(4):1494–1508. doi: 10.1128/jb.91.4.1494-1508.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silengo L., Schlessinger D., Mangiarotti G., Apirion D. Induction of mutations to streptomycin and spectinomycin resistance in Escherichia coli by N-methyl-N'-nitroso-N-nitroguanidine and acridine half-mustard ICR-191. Mutat Res. 1967 Sep-Oct;4(5):701–703. doi: 10.1016/0027-5107(67)90056-5. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



