Abstract
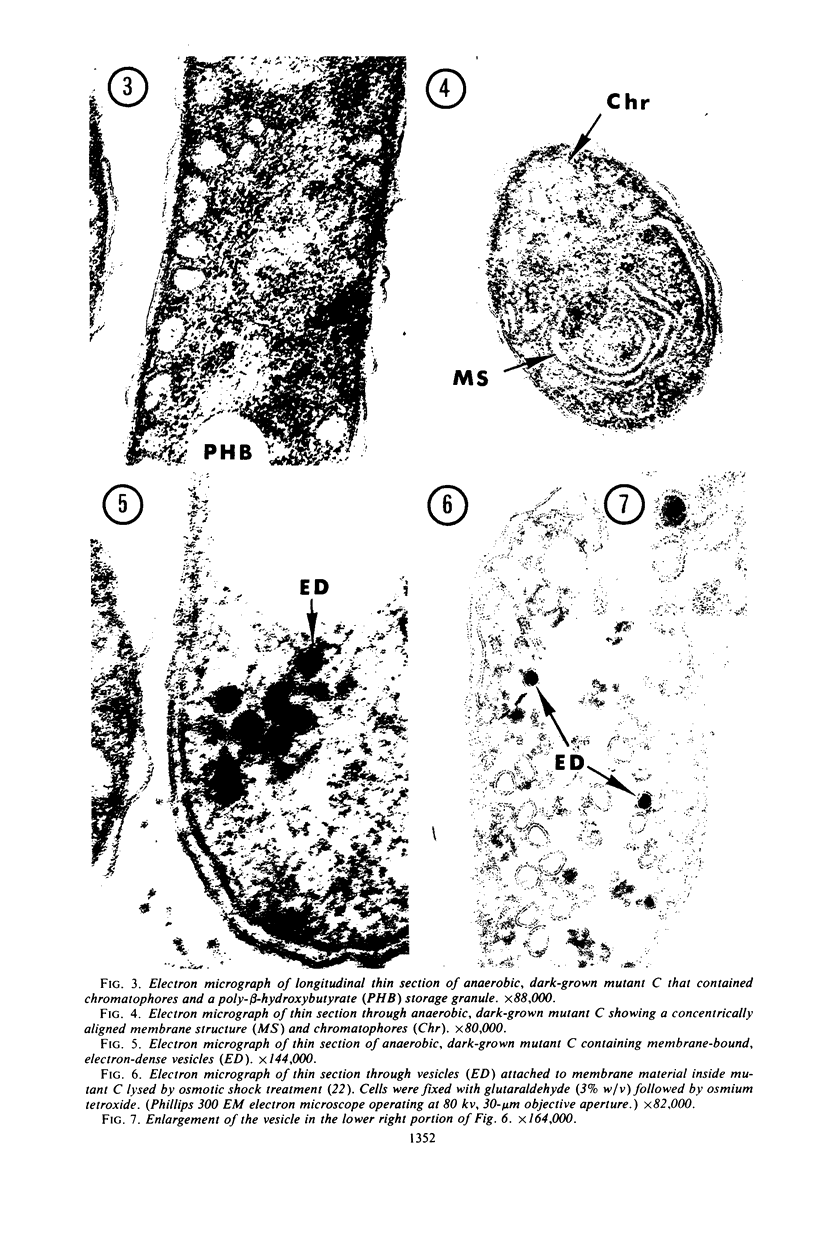
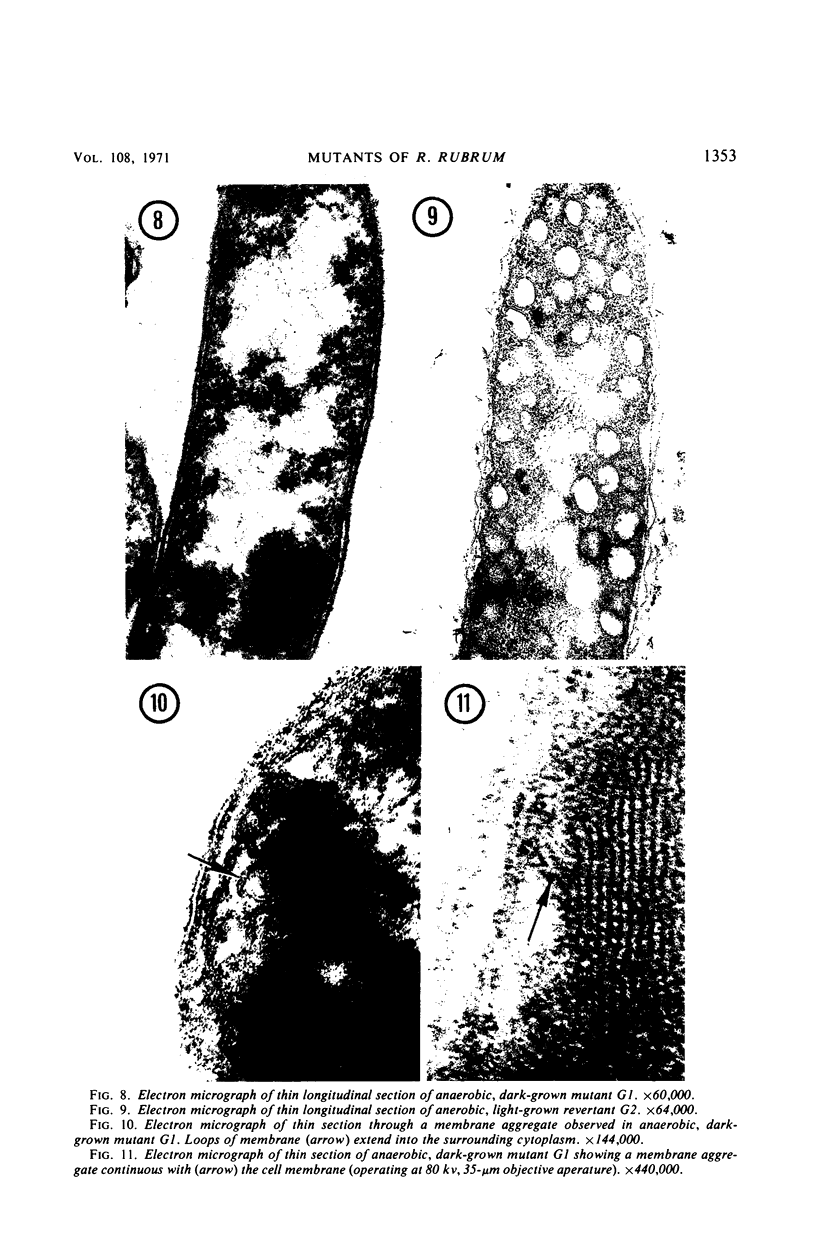
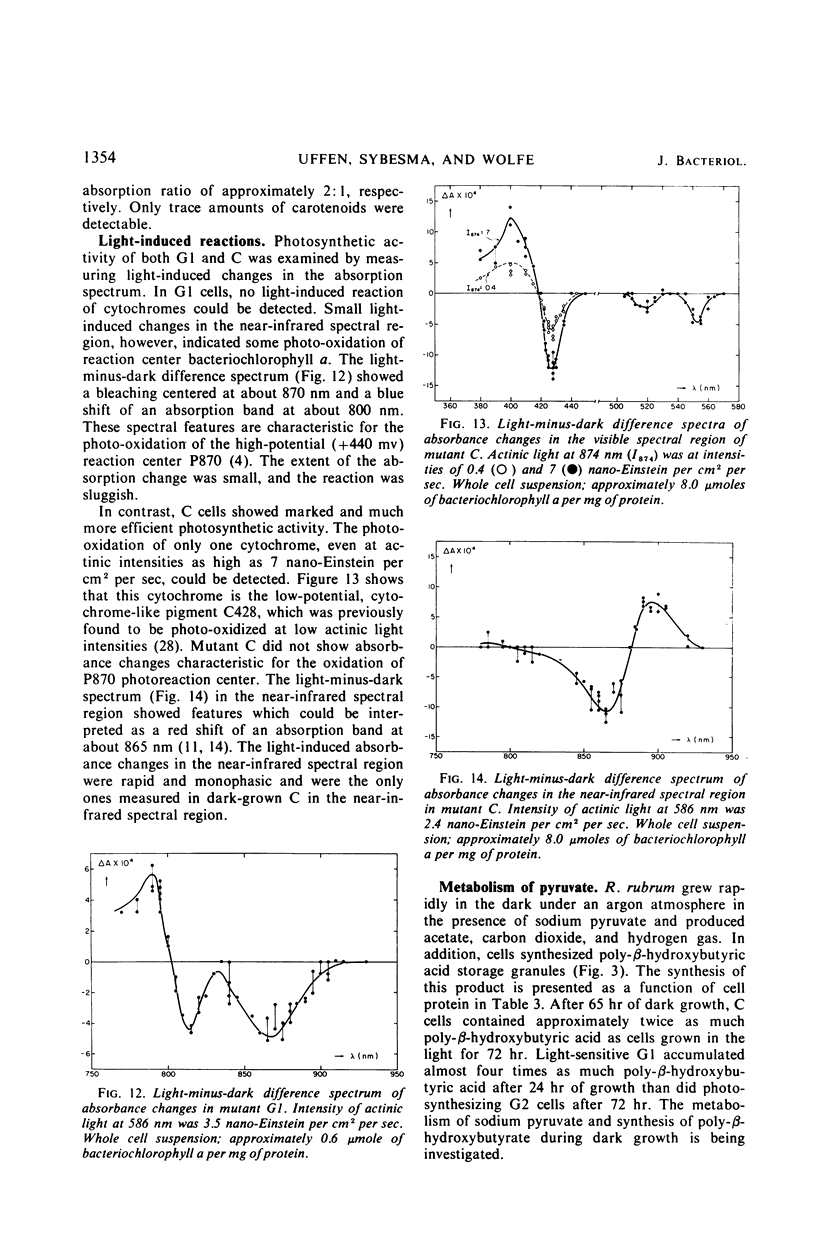
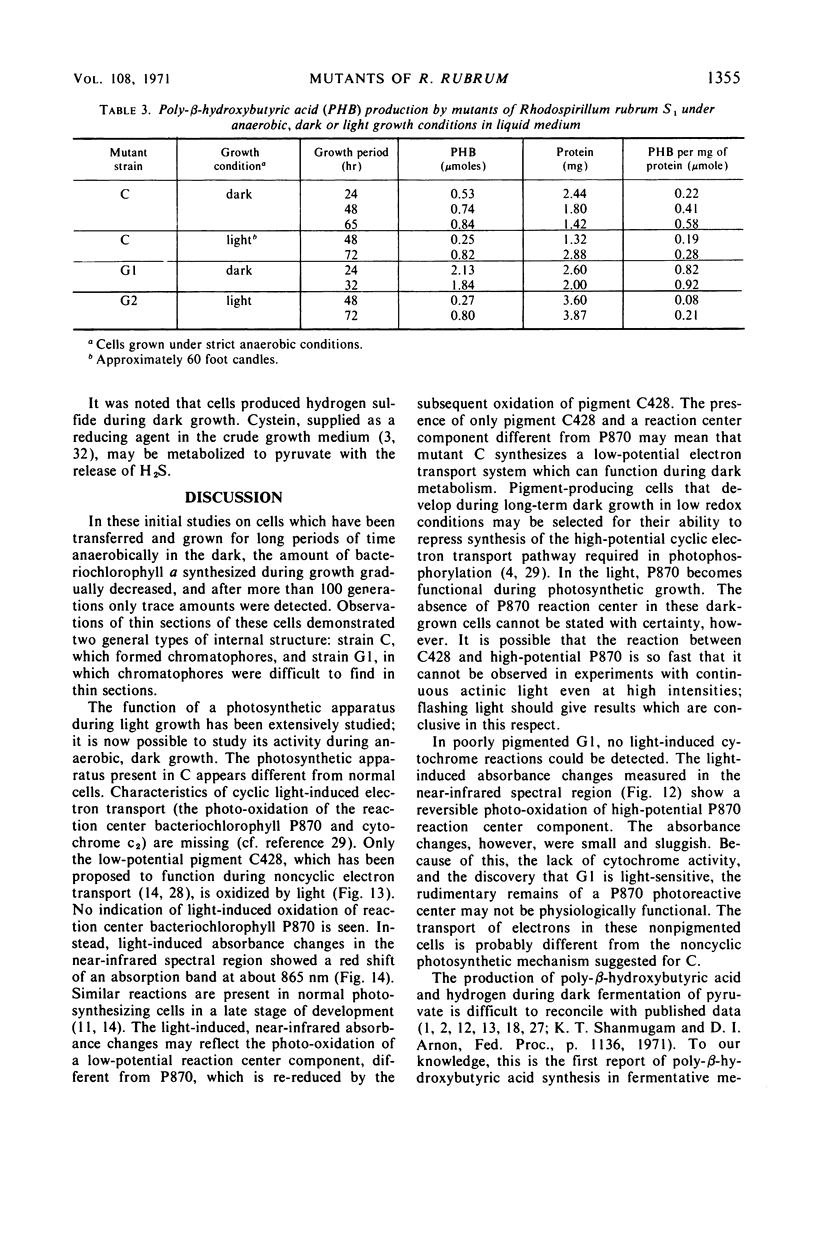
Rhodospirillum rubrum S1 cells were grown for more than 100 generations under strict anaerobic, dark conditions in liquid medium with sodium pyruvate. During this time, growth became nonpigmented. When cells were streaked onto the surface of solid growth medium in anaerobic bottles and placed in the dark, a few light-red colonies developed, but the majority was nonpigmented. Mutants were obtained from colonies selected on the basis of pigmentation and bacteriochlorophyll a content. The growth, ultrastructure, and light reactivity of two mutants were examined. Mutant C synthesized bacteriochlorophyll a (7.2 μmoles per mg of protein), altered membrane structures, and chromatophores during dark growth. Examination of light-induced changes of the absorption spectrum of this mutant suggested that only an electron transport pathway, which included the low potential cytochrome-like pigment C428, could be detected. Mutant C grew anaerobically in the light, whereas mutant G1 was light sensitive and produced only trace amounts of bacteriochlorophyll a (0.6 μmole per ml of protein). Poorly pigmented G1 cells contained unusual membrane structures. When dark-grown G1 colonies were placed in the light, deep-red colored papillae developed in the nonpigmented colonies. During anaerobic, dark growth with sodium pyruvate, both C and G1 synthesized poly-β-hydroxybutyrate and produced acetate, carbon dioxide, and hydrogen gas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENNETT R., RIGOPOULOS N., FULLER R. C. THE PYRUVATE PHOSPHOROCLASTIC REACTION AND LIGHT-DEPENDENT NITROGEN FIXATION IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1964 Sep;52:762–768. doi: 10.1073/pnas.52.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshard-Heer E., Bachofen R. Synthese von Specicherstoffen aus Pyruvat durch Rhodospirillum rubrum. Arch Mikrobiol. 1969;65(1):61–75. [PubMed] [Google Scholar]
- CLAYTON R. K. TOWARD THE ISOLATION OF A PHOTOCHEMICAL REACTION CENTER IN RHODOPSEUDOMONAS SPHEROIDES. Biochim Biophys Acta. 1963 Nov 29;75:312–323. doi: 10.1016/0006-3002(63)90618-8. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., London J. Basal organelles of bacterial flagella. J Bacteriol. 1967 Aug;94(2):458–465. doi: 10.1128/jb.94.2.458-465.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. SOME ASPECTS OF THE ENDOGENOUS METABOLISM OF BACTERIA. Bacteriol Rev. 1964 Jun;28:126–149. doi: 10.1128/br.28.2.126-149.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felter R. A., Kennedy S. F., Colwell R. R., Chapman G. B. Intracytoplasmic membrane structures in Vibrio marinus. J Bacteriol. 1970 May;102(2):552–560. doi: 10.1128/jb.102.2.552-560.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. F., Sybesma C. Light- and chemically-induced oxidation-reduction reactions in chromatophore fractions of Rhodospirillum rubrum. Biochim Biophys Acta. 1970 Mar 3;197(2):276–283. doi: 10.1016/0005-2728(70)90038-1. [DOI] [PubMed] [Google Scholar]
- GEST H., ORMEROD J. G., ORMEROD K. S. Photometabolism of Rhodospirillum rubrum: light-dependent dissimilation of organic compounds to carbon dioxide and molecular hydrogen by an anaerobic citric acid cycle. Arch Biochem Biophys. 1962 Apr;97:21–33. doi: 10.1016/0003-9861(62)90039-5. [DOI] [PubMed] [Google Scholar]
- GEST H. Properties of cell-free hydrogenases of Escherichia coli and Rhodospirillum rubrum. J Bacteriol. 1952 Jan;63(1):111–121. doi: 10.1128/jb.63.1.111-121.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Sybesma C. Light-induced reduction of pyridine nucleotide and its relation to light-induced electron transport in whole cells of Rhodospirillum rubrum. Biochim Biophys Acta. 1970 Dec 8;223(2):251–260. doi: 10.1016/0005-2728(70)90182-9. [DOI] [PubMed] [Google Scholar]
- HICKMAN D. D., FRENKEL A. W. The structure of Rhodospirillum rubrum. J Biophys Biochem Cytol. 1959 Oct;6:277–284. doi: 10.1083/jcb.6.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHLMILLER E. F., Jr, GEST H. A comparative study of the light and dark fermentations of organic acids by Rhodo-spirillum rubrum. J Bacteriol. 1951 Mar;61(3):269–282. doi: 10.1128/jb.61.3.269-282.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Oppenheim J., Marcus L. Correlation of ultrastructure in Azotobacter vinelandii with nitrogen source for growth. J Bacteriol. 1970 Jan;101(1):286–291. doi: 10.1128/jb.101.1.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N. Photosynthetic bacteria. Annu Rev Microbiol. 1967;21:285–324. doi: 10.1146/annurev.mi.21.100167.001441. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Rhodopseudomonas acidophila, sp. n., a new species of the budding purple nonsulfur bacteria. J Bacteriol. 1969 Aug;99(2):597–602. doi: 10.1128/jb.99.2.597-602.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. L., Kamen M. D. Variable cellular composition of Chromatium in browing cultures. Arch Mikrobiol. 1970;73(1):1–18. doi: 10.1007/BF00409947. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Doudoroff M., Kunisawa R., Contopoulou R. THE ROLE OF ORGANIC SUBSTRATES IN BACTERIAL PHOTOSYNTHESIS. Proc Natl Acad Sci U S A. 1959 Aug;45(8):1246–1260. doi: 10.1073/pnas.45.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sybesma C., Fowler C. F. Evidence for two light-driven reactions in the purple photosynthetic bacterium, Rhodospirillum rubrum. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1343–1348. doi: 10.1073/pnas.61.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauschel H. D., Drews G. Zum Vorkommen helifxförmig angeordneter Ribosomen bei Rhodopseudomonas palustris. Arch Mikrobiol. 1969;64(4):377–386. [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand R. A., Shively J. M., Greenawalt J. W. Formation and ultrastructure of extra membranes in Escherichia coli. J Bacteriol. 1970 Apr;102(1):240–249. doi: 10.1128/jb.102.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]