Abstract
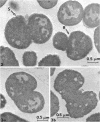
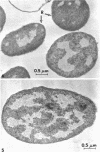
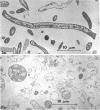
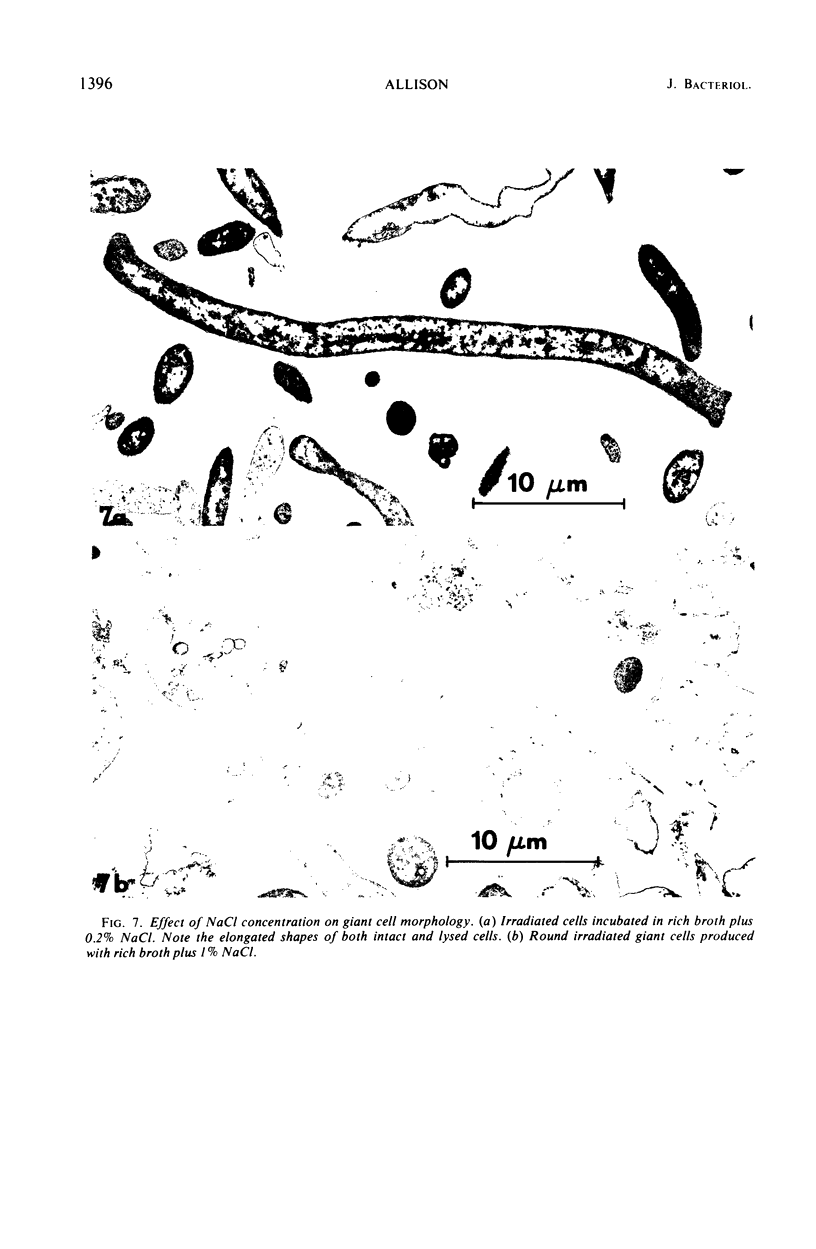
Bacterial growth without division was observed in a giant cell-producing strain of Escherichia coli K-12. Giant cell production is controlled by the lon− (failure of cell division after irradiation) and mon− (formation of irregularly shaped cells) genes. Irradiation of a lon−mon− strain (P678-A4) with low doses of ultraviolet or ionizing radiation results in the production of large, amorphous giant cells with 500 to 1,000 times the volume of the nonirradiated parents. The concentration of NaCl in the growth medium was found to influence irradiated-cell morphology. Low concentrations (0.2% NaCl) resulted in elongated cells, whereas spherical giant cells were produced in the presence of high salt (1% NaCl) concentrations.
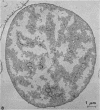
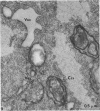
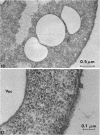
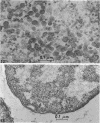
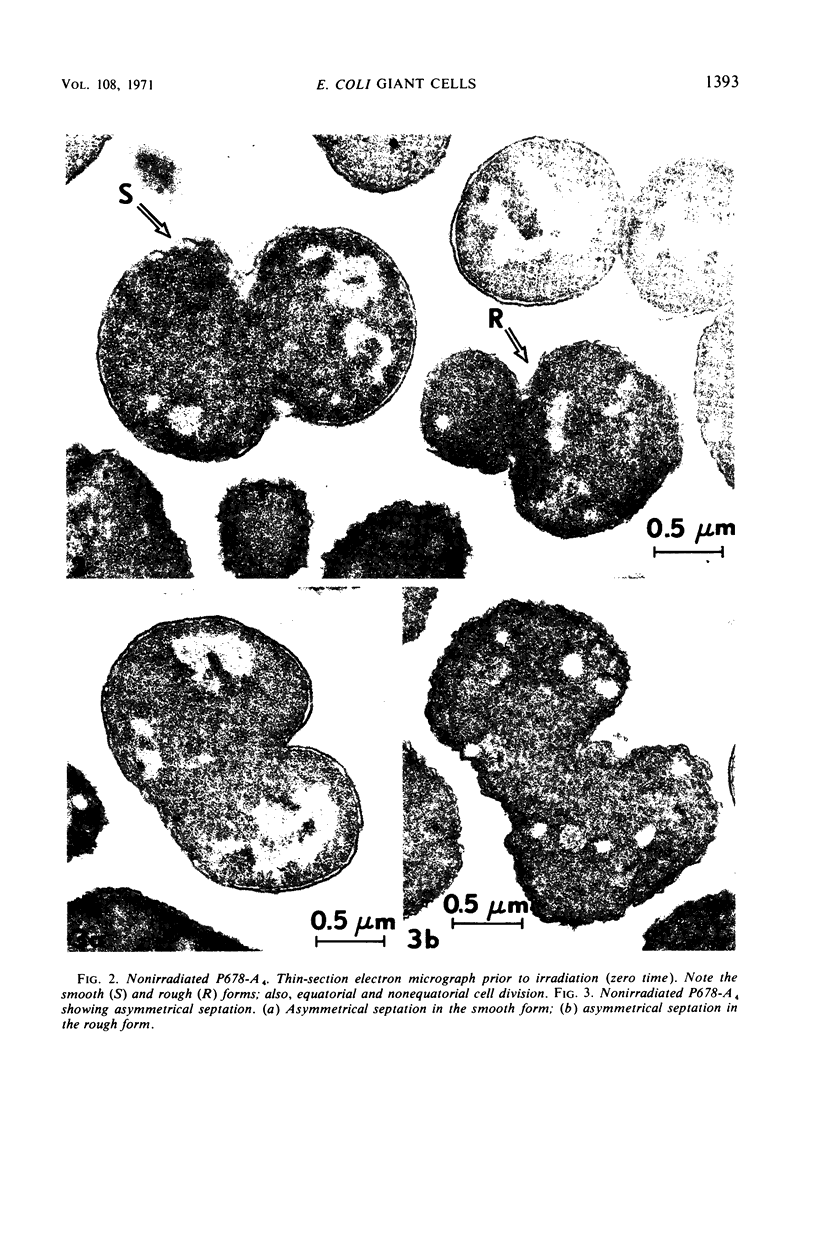
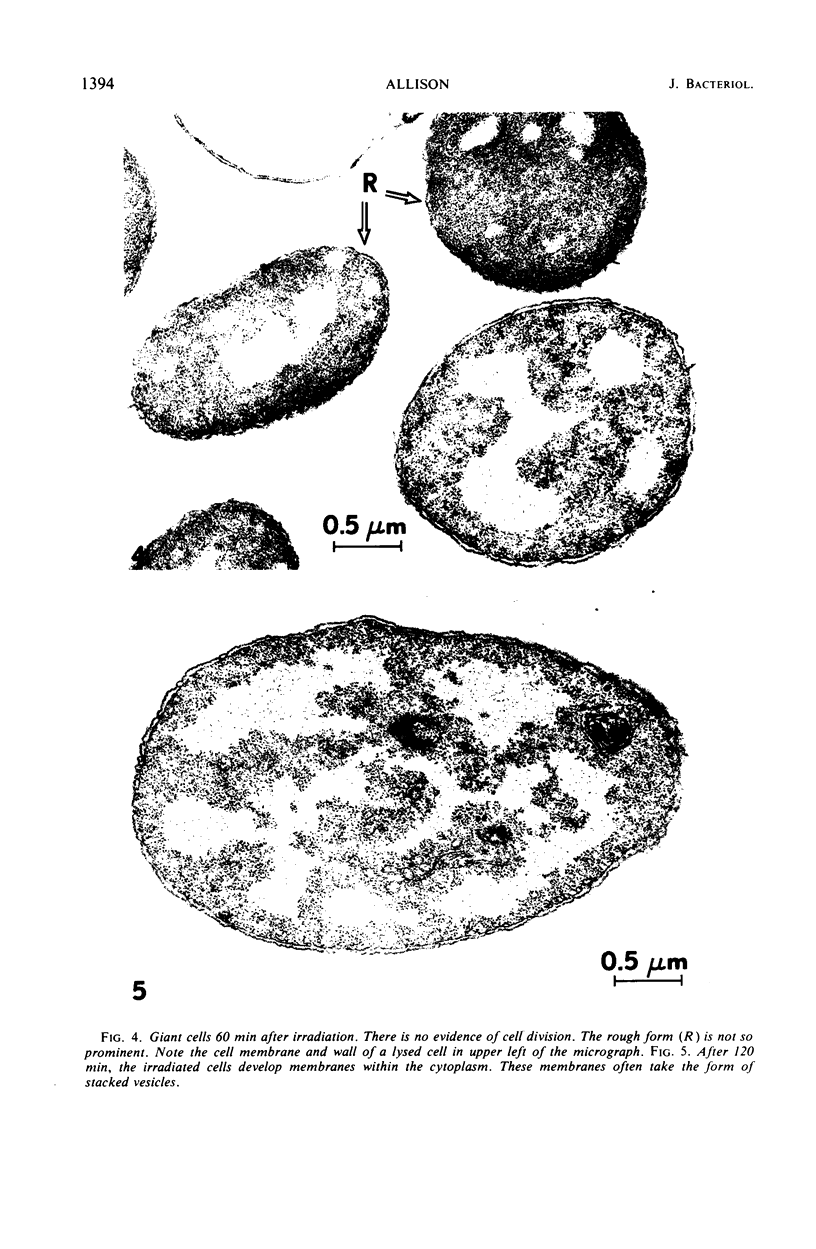
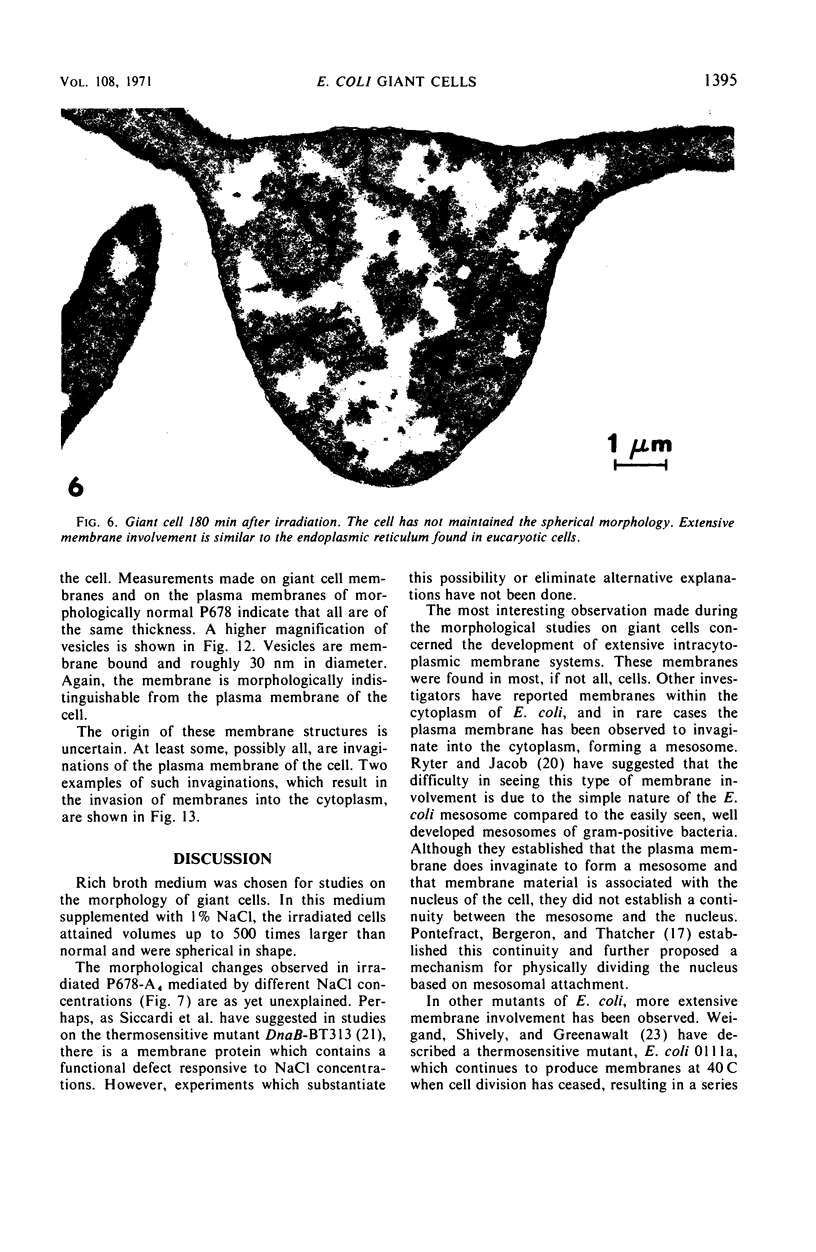
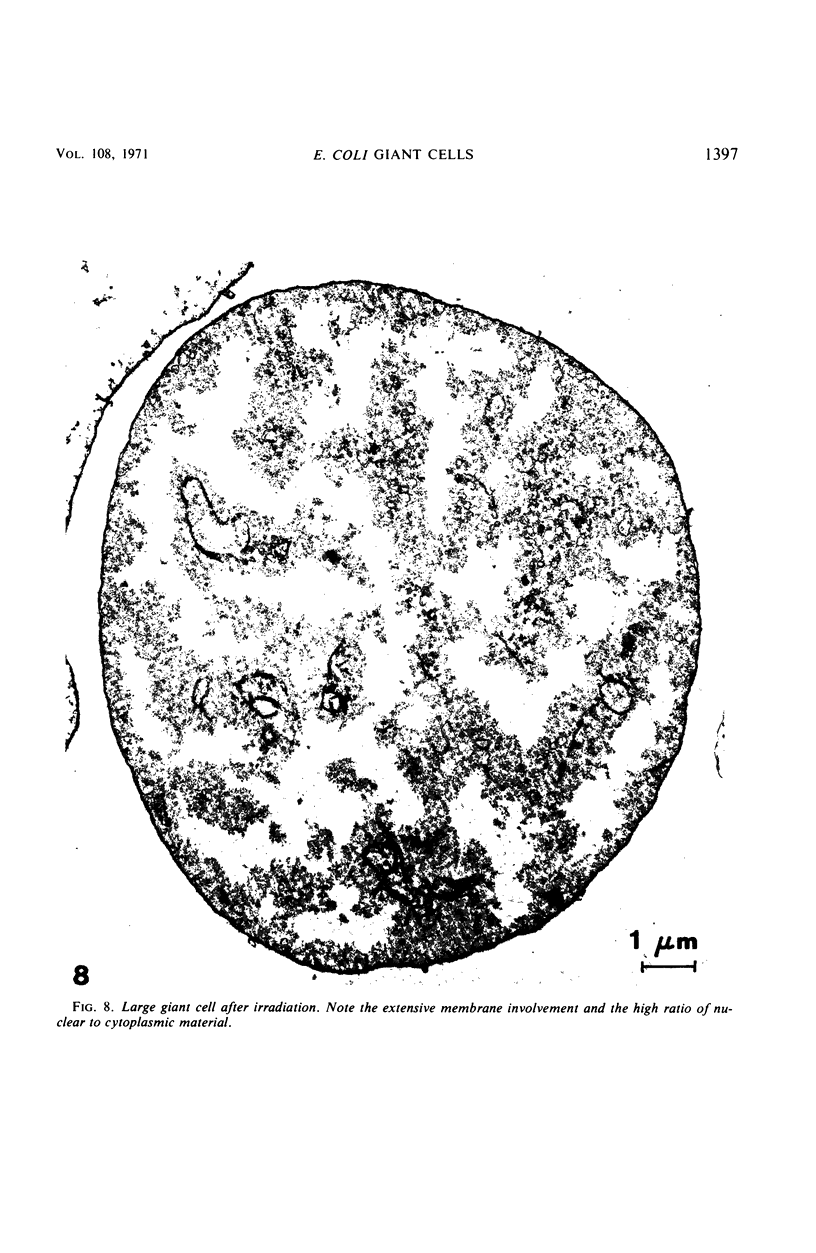
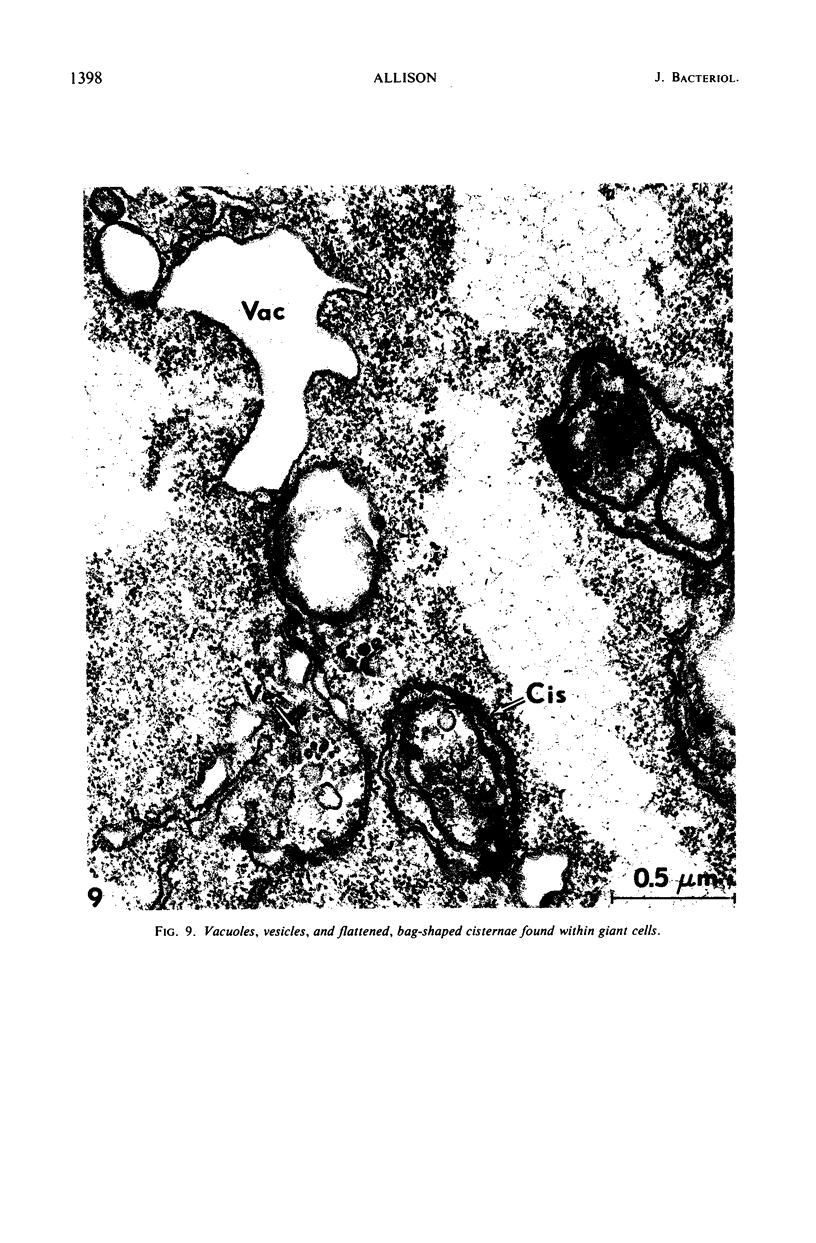
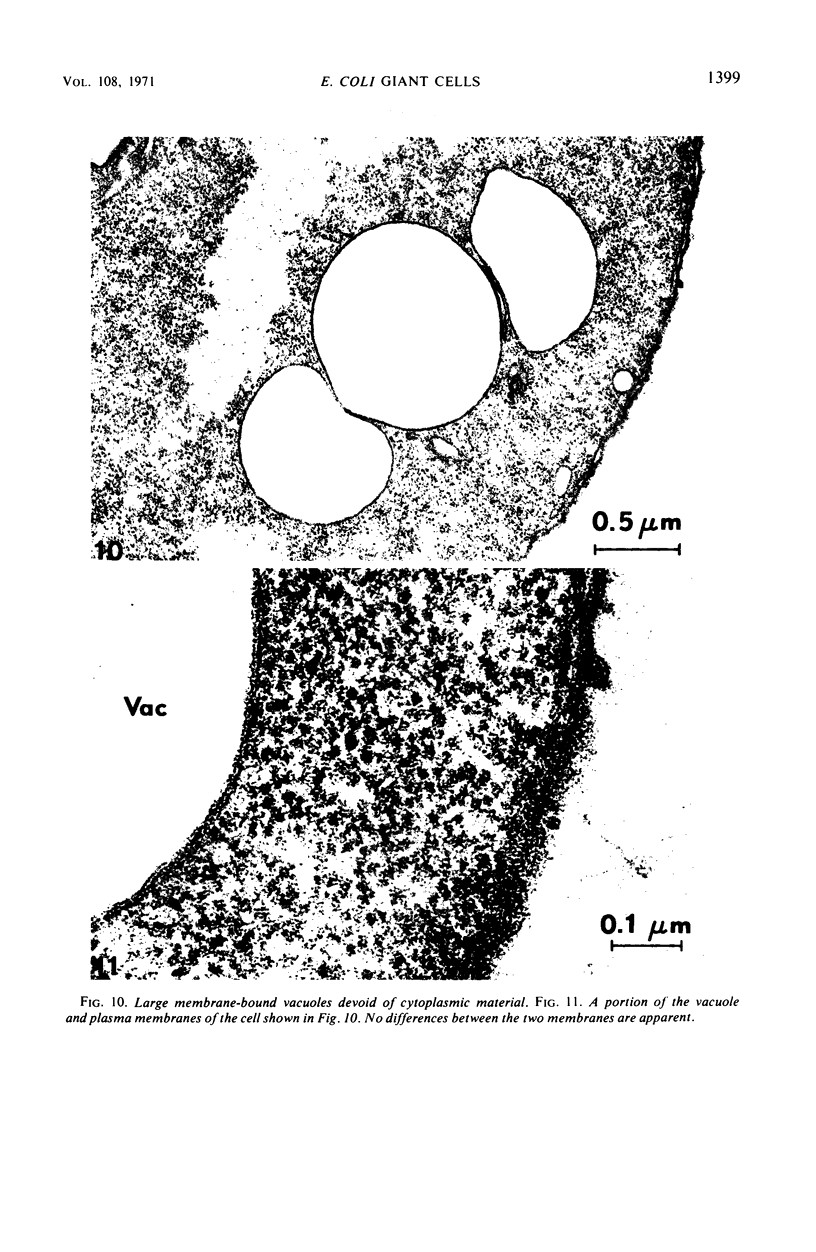
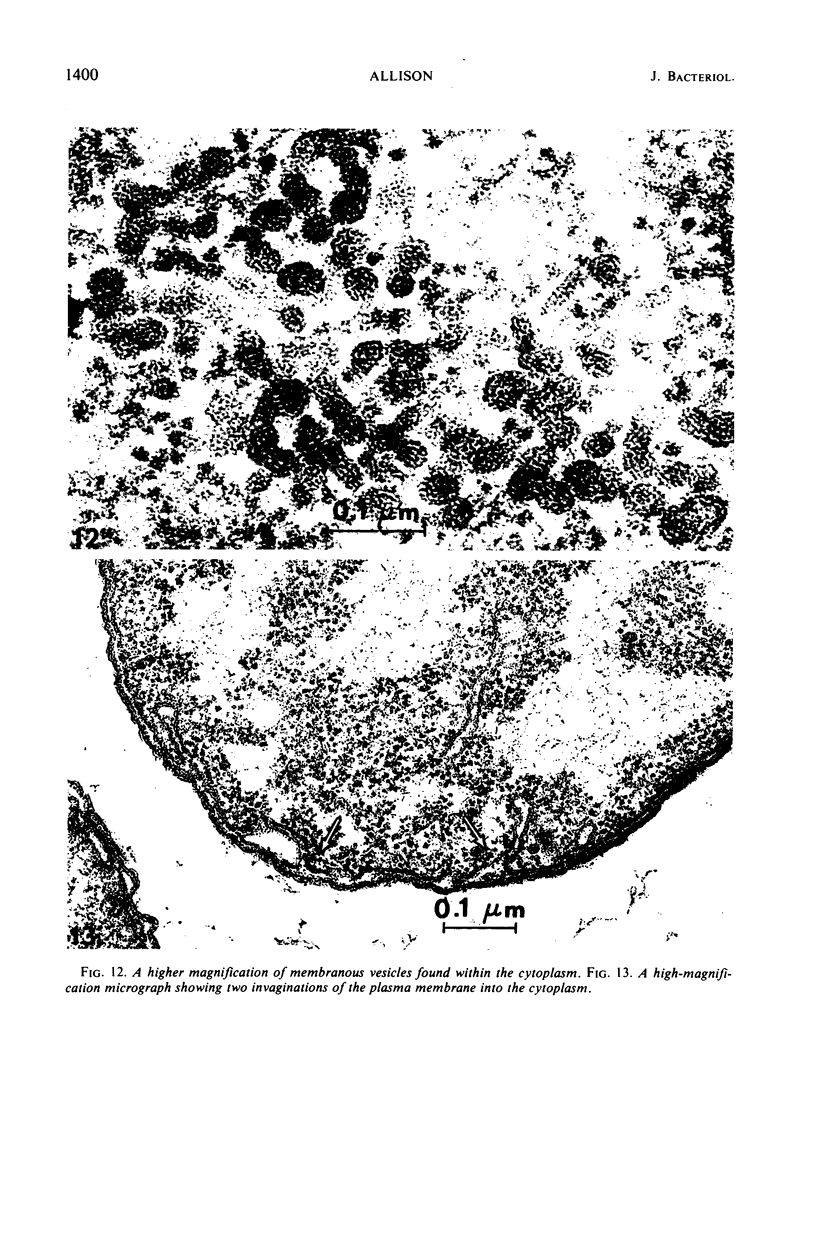
Thin-section electron microscopy revealed an extensive network of intracellular membranes forming vacuoles, vesicles, and cisternae. These structures bear a striking resemblance to the rough and smooth membranes (endoplasmic reticulum, Golgi complex, vacuoles, etc.) found in eucaryotic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER H. I., COPELAND J. C. Genetic analysis of radiation response in Escherichia coli. Genetics. 1962 Jun;47:701–712. doi: 10.1093/genetics/47.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER H. I., HARDIGREE A. A. ANALYSIS OF A GENE CONTROLLING CELL DIVISION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Mar;87:720–726. doi: 10.1128/jb.87.3.720-726.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler H. I., Terry C. E., Hardigree A. A. Giant cells of Escherichia coli. J Bacteriol. 1968 Jan;95(1):139–142. doi: 10.1128/jb.95.1.139-142.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischman D. A., Weinbaum G. The formation of multiple layers of membrane-like structures in Escherichia coli B. J Cell Biol. 1967 Feb;32(2):524–528. doi: 10.1083/jcb.32.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBERG J. A LOCUS FOR RADIATION RESISTANCE IN ESCHERICHIA COLI. Genetics. 1964 May;49:771–778. doi: 10.1093/genetics/49.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY A. D. An upper limit to the protein content of the germinal substance of bacteriophage T2. Virology. 1955 May;1(1):108–127. doi: 10.1016/0042-6822(55)90009-x. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Inouye M., Pardee A. B. Requirement of polyamines for bacterial division. J Bacteriol. 1970 Mar;101(3):770–776. doi: 10.1128/jb.101.3.770-776.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAGGER J. A small and inexpensive ultraviolet dose-rate meter useful in biological experiements. Radiat Res. 1961 Apr;14:394–403. [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontefract R. D., Bergeron G., Thatcher F. S. Mesosomes in Escherichia coli. J Bacteriol. 1969 Jan;97(1):367–375. doi: 10.1128/jb.97.1.367-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Jacob F. Etude morphologique de la liaison du noyau à la membrane chez E. coli et chez les protoplastes de B. subtilis. Ann Inst Pasteur (Paris) 1966 Jun;110(6):801–812. [PubMed] [Google Scholar]
- Siccardi A. G., Shapiro B. M. On the process of cellular division in Escherichia coli. IV. Altered protein composition and turnover of the membranes of thermosensitive mutants defective in chromosomal replication. J Mol Biol. 1971 Mar 28;56(3):475–490. doi: 10.1016/0022-2836(71)90395-0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weigand R. A., Shively J. M., Greenawalt J. W. Formation and ultrastructure of extra membranes in Escherichia coli. J Bacteriol. 1970 Apr;102(1):240–249. doi: 10.1128/jb.102.1.240-249.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]