Abstract
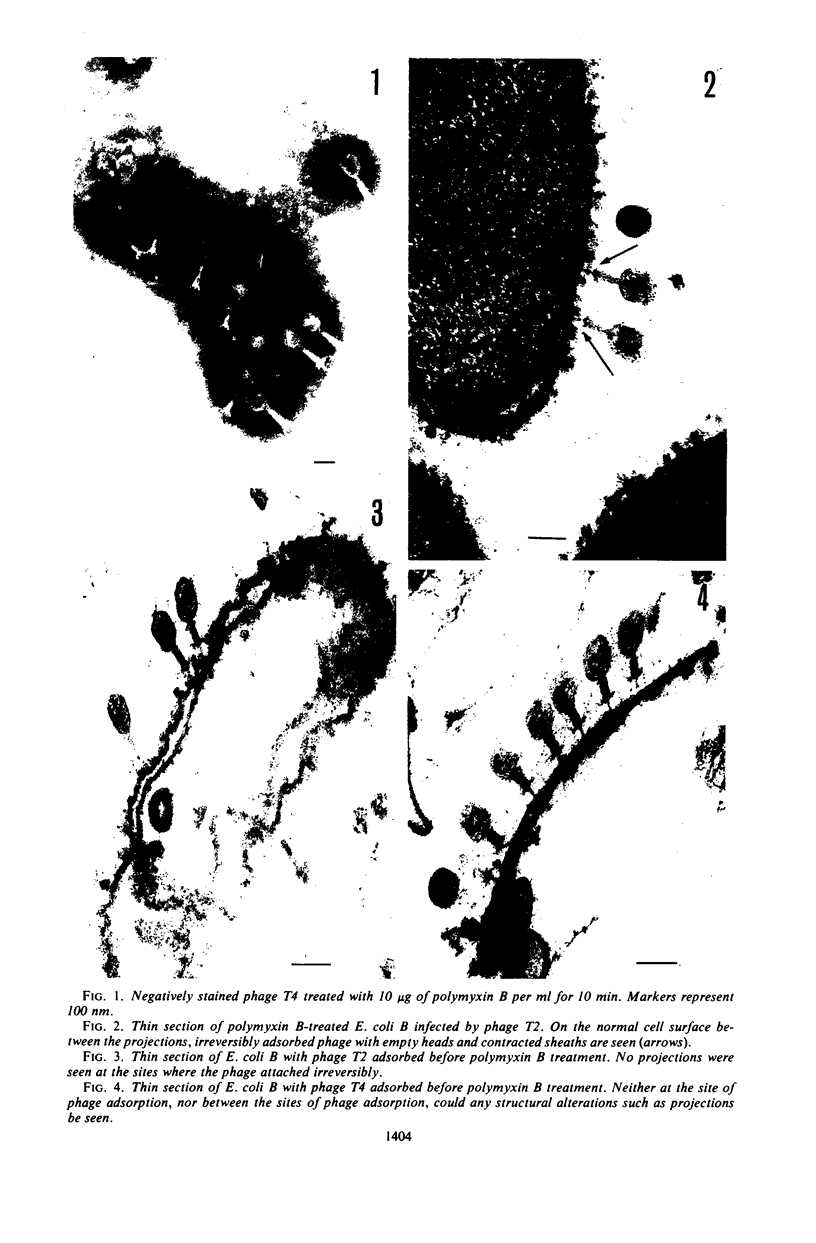
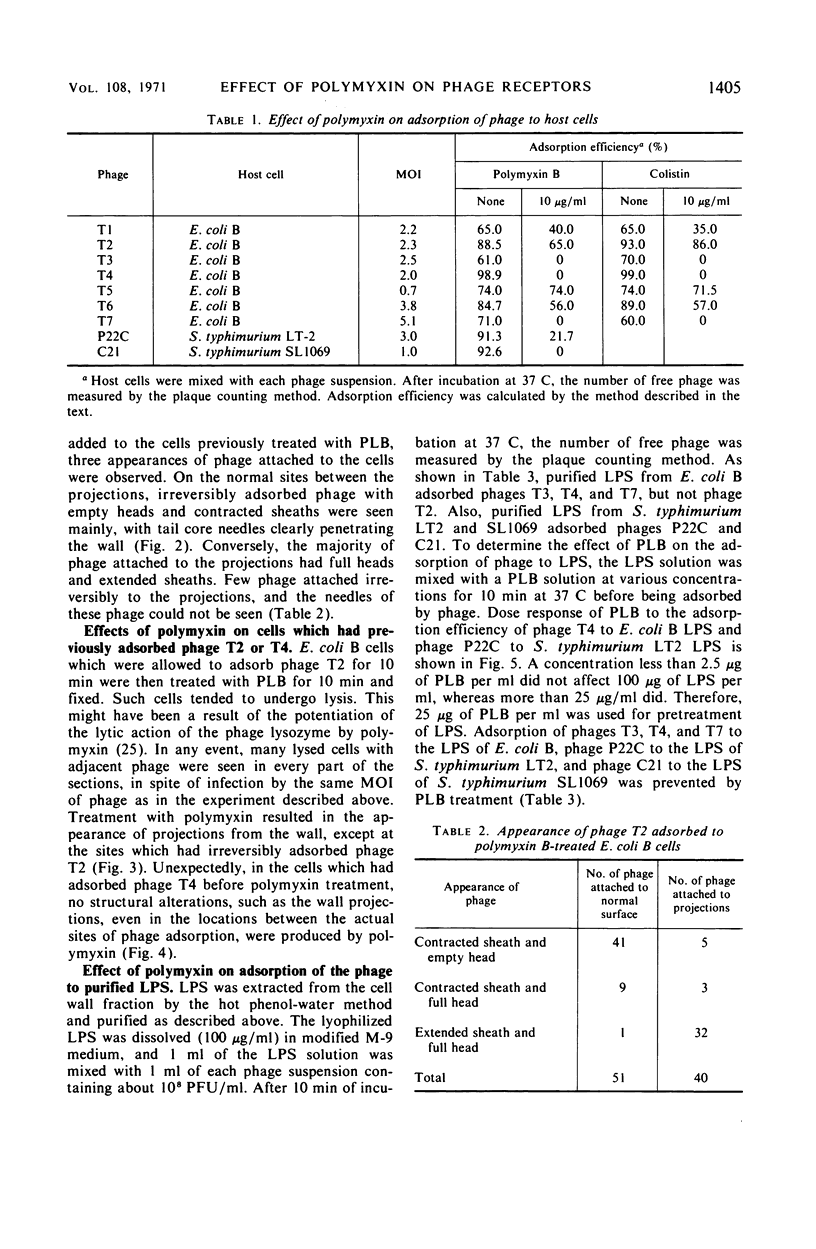
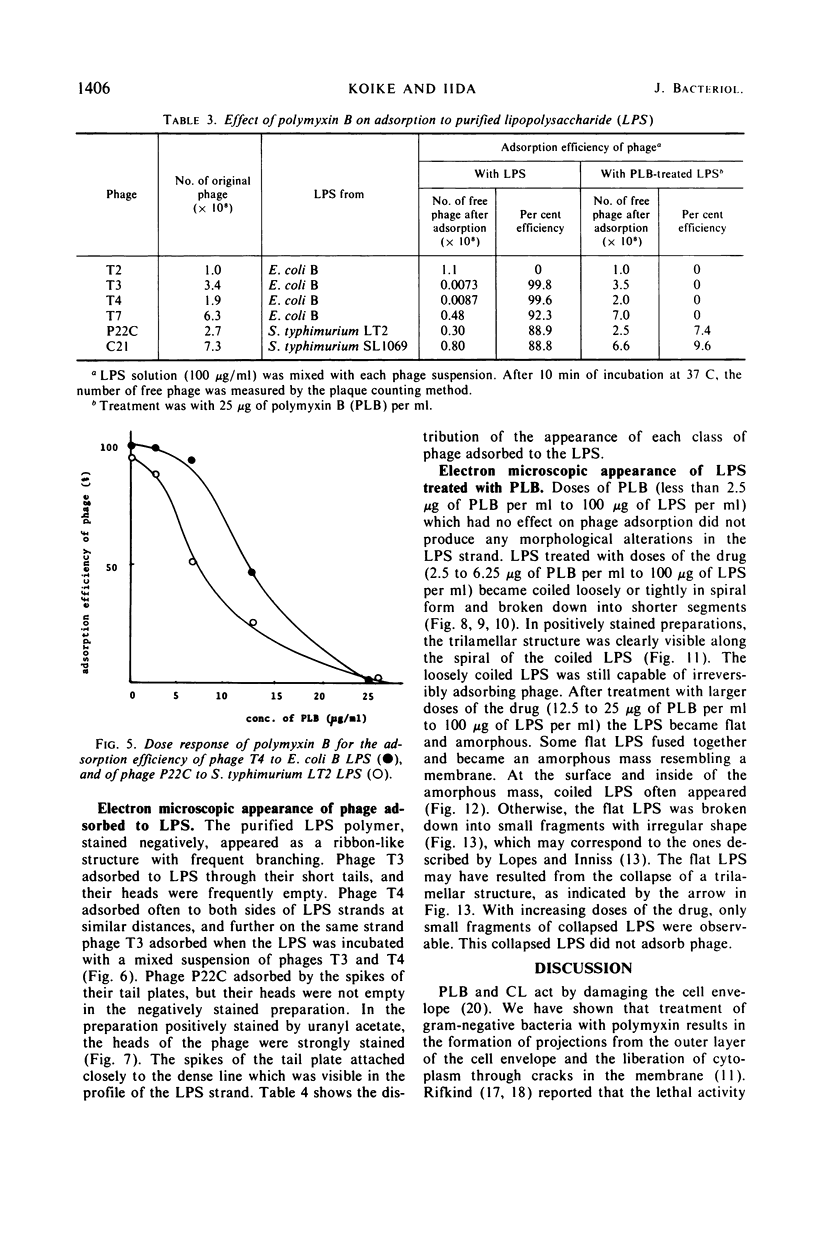
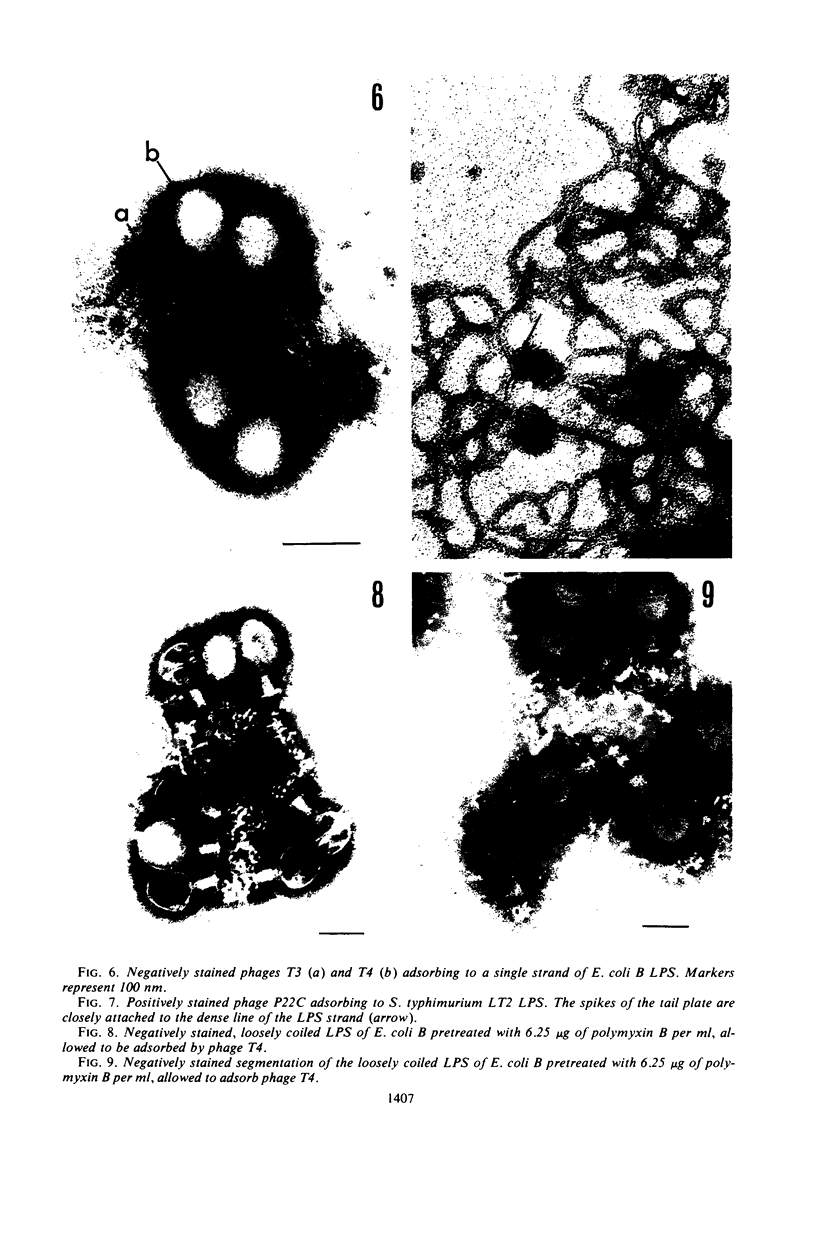
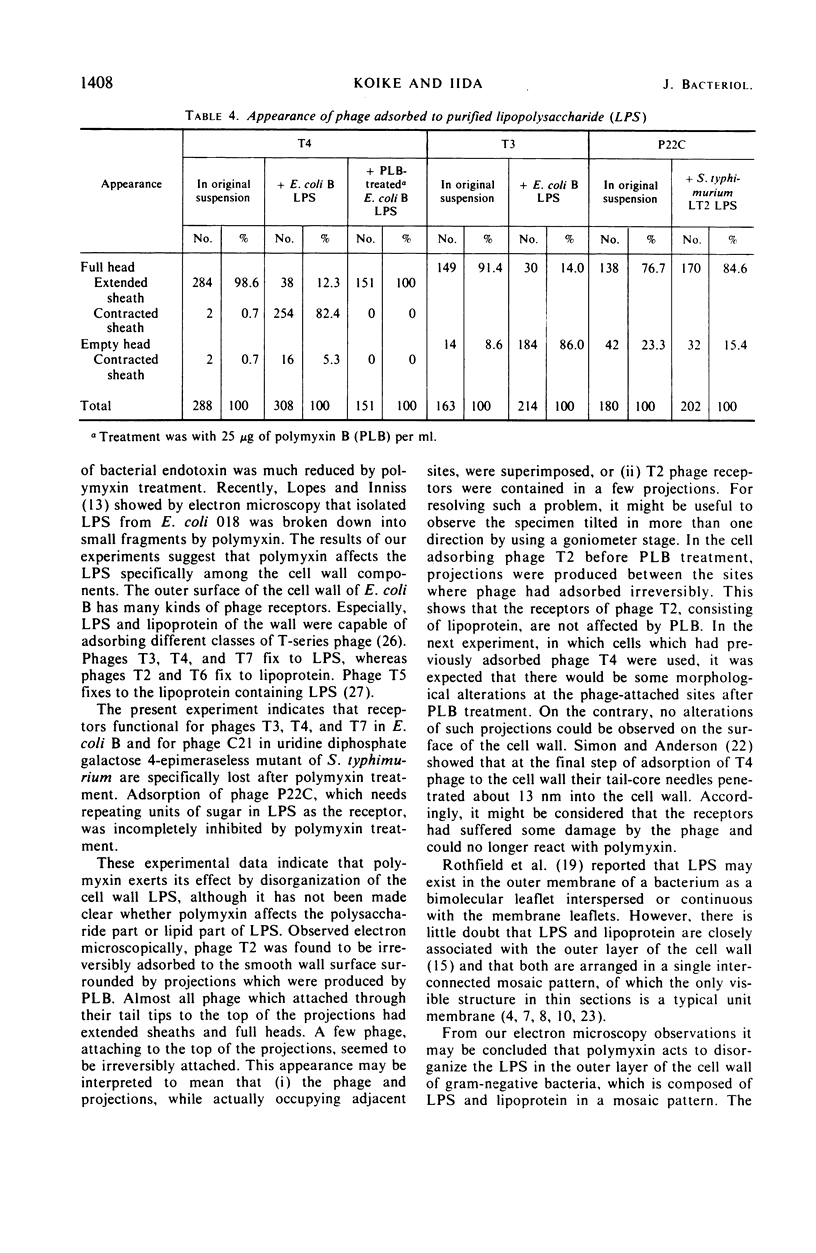
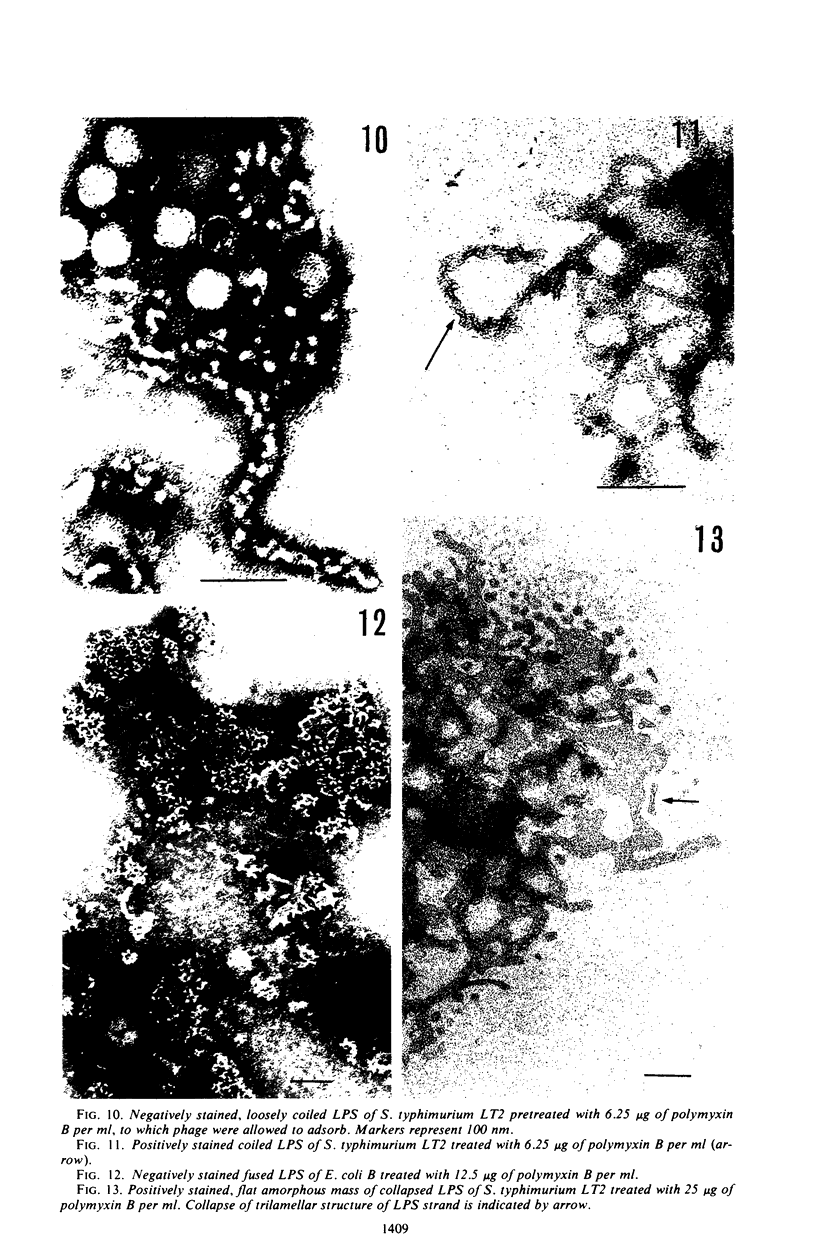
Treatment of gram-negative bacteria with lethal doses of polymyxin B and colistin resulted in the formation of projections of the outer layer of the cell wall. Phages T3, T4, and T7, which use wall lipopolysaccharide as receptors, were specifically prevented from adsorbing to Escherichia coli B cells treated with polymyxin, whereas phages T1, T2, T5, and T6 were not. In the systems of phage P22C-Salmonella typhimurium LT2 and phage C21-S. typhimurium variant SL1069, the phage were prevented from adsorbing to the host cell treated with the antibiotics. Electron microscopic observations show that phage T2 adsorbed irreversibly to the normal smooth surface between the projections on the outer layer caused by the drug treatment. These results indicate that lipopolysaccharide is affected by polymyxin functionally and morphologically, but lipoprotein is not. The purified lipopolysaccharide showed a ribbon-like structure when viewed face on and showed trilamellar structure when viewed edge on. The lipopolysaccharide from E. coli B was irreversibly adsorbed by phages T3, T4, and T7, but not phage T2. Often, phage T4 adsorbed to both sides of the lipopolysaccharide strand at comparable distances. Phage P22C adsorbed through the spikes of the tail-plates to the lipopolysaccharide from S. typhimurium LT2. Lipopolysaccharide which was treated with low doses of the drug (2.5 to 6.25 μg of polymyxin B per ml to 100 μg of lipopolysaccharide per ml) turned into the coiled form and was partially broken down into short segments with coiled form. The loosely coiled lipopolysaccharide retains both its function as the receptor and its trilamellar structure. Treatment with high doses of the drug (12.5 to 25 μg of polymyxin B per ml to 100 μg of lipopolysaccharide per ml) caused the collapse of the trilamellar structure of the strand. These collapsed lipopolysaccharides became flat and fused with each other, making an amorphous mass, and finally they were broken into small collapsed fragments.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. I. Cytological changes associated with the exposure of Escherichia coli to colistin sulfate. J Bacteriol. 1962 Jul;84:169–179. doi: 10.1002/path.1700840118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B. Cytological aspects of antimicrobial antibiosis. II. Cytological changes associated with the exposure of Pseudomonas aeruginosa and Bacillus megaterium to colistin sulfate. J Bacteriol. 1962 Jul;84:180–185. doi: 10.1128/jb.84.1.180-185.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- FEW A. V., SCHULMAN J. H. Absorption of polymyxin by bacteria. Nature. 1953 Apr 11;171(4354):644–645. doi: 10.1038/171644a0. [DOI] [PubMed] [Google Scholar]
- FEW A. V., SCHULMAN J. H. The absorption of polymyxin E by bacteria and bacterial cell walls and its bactericidal action. J Gen Microbiol. 1953 Dec;9(3):454–466. doi: 10.1099/00221287-9-3-454. [DOI] [PubMed] [Google Scholar]
- Frank H., Dekegel D. Electron microscopical studies on the localisation of the different components of cell walls of gram-negative bacteria. Folia Microbiol (Praha) 1967;12(3):227–233. doi: 10.1007/BF02868736. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. The release of soluble constituents from washed cells of Pseudomonas aeruginosa by the action of polymyxin. J Gen Microbiol. 1953 Aug;9(1):54–64. doi: 10.1099/00221287-9-1-54. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Palmer J. D. Neutralization of endotoxin toxicity in chick embryos by antibiotics. J Bacteriol. 1966 Oct;92(4):815–819. doi: 10.1128/jb.92.4.815-819.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967 Apr;93(4):1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Takeshita M., Pearlman M., Horne R. W. Role of phospholipids in the enzymatic synthesis of the bacterial cell envelope. Fed Proc. 1966 Sep-Oct;25(5):1495–1502. [PubMed] [Google Scholar]
- Shands J. W., Jr, Graham J. A., Nath K. The morphologic structure of isolated bacterial lipopolysaccharide. J Mol Biol. 1967 Apr 14;25(1):15–21. doi: 10.1016/0022-2836(67)90275-6. [DOI] [PubMed] [Google Scholar]
- Simon L. D., Anderson T. F. The infection of Escherichia coli by T2 and T4 bacteriophages as seen in the electron microscope. I. Attachment and penetration. Virology. 1967 Jun;32(2):279–297. doi: 10.1016/0042-6822(67)90277-2. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M. Fine structure and radiation resistance in Acinetobacter: studies on a resistant strain. J Cell Sci. 1968 Jun;3(2):273–294. doi: 10.1242/jcs.3.2.273. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN G. H., GRAY J., YURCHENCO J. A. Effect of polymyxin on the lysis of Neisseria catarrhalis by lysozyme. J Bacteriol. 1957 Dec;74(6):788–793. doi: 10.1128/jb.74.6.788-793.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W. Bacterial viruses; with particular reference to adsorption/penetration. Annu Rev Microbiol. 1958;12:27–48. doi: 10.1146/annurev.mi.12.100158.000331. [DOI] [PubMed] [Google Scholar]