Abstract
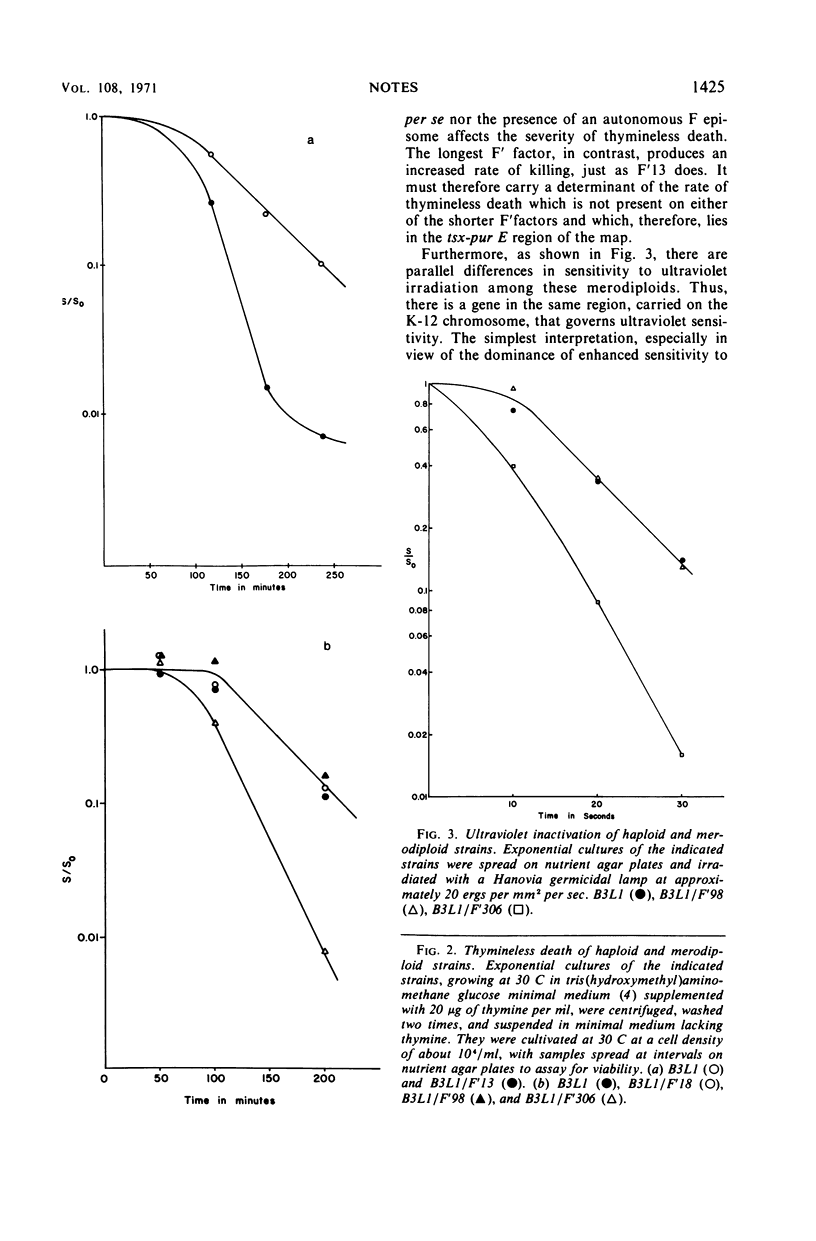
We have infected Escherichia coli strain B3 with F′ factors carrying different lengths of the K-12 chromosome. When the F′ factor carries the tsx to purE segment, the resulting hybrid merodiploid shows increased sensitivity to thymineless death, although leaky deoxyribonucleic acid synthesis is unaffected, as well as increased sensitivity to ultraviolet irradiation. The dominant or partially dominant character of the effect indicates that it is not a product of allelic differences between E. coli K and B at either ras or lon.
Full text
PDF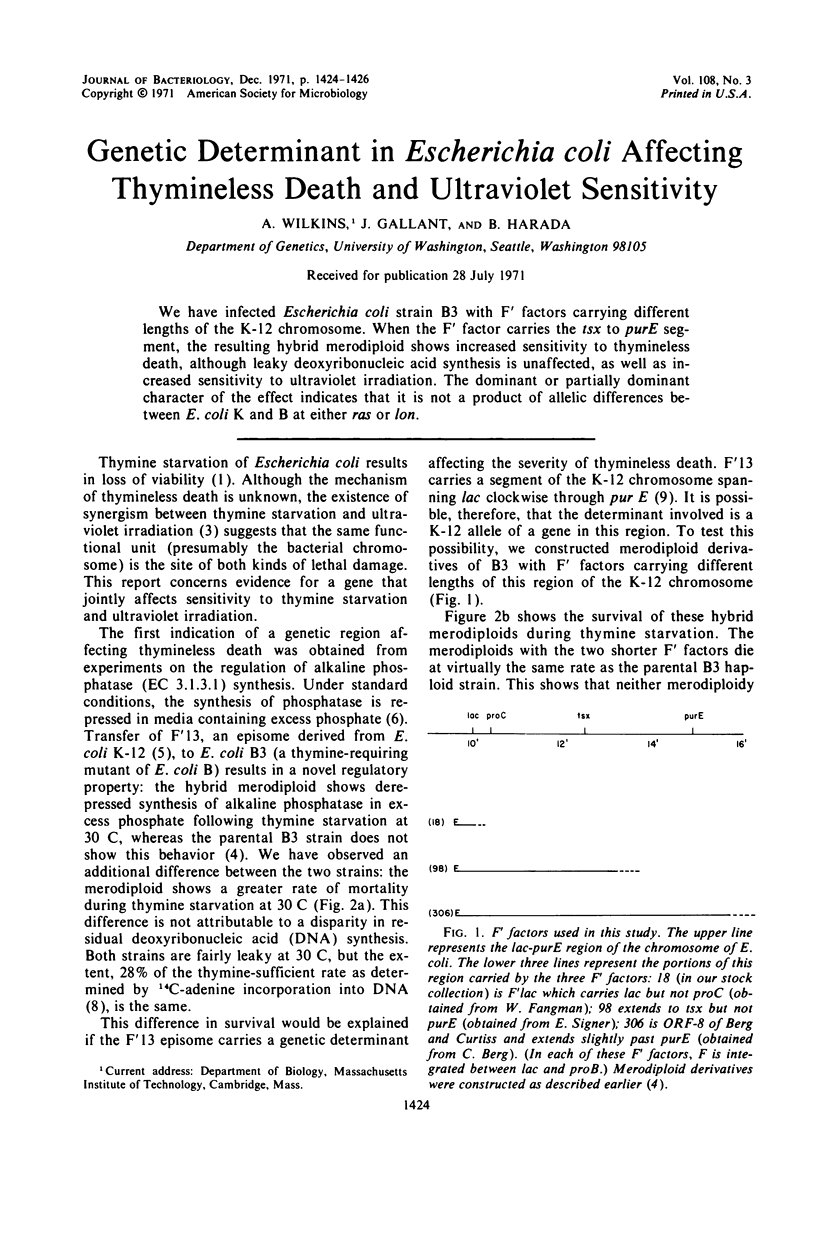

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARNER H. D., COHEN S. S. The induction of thymine synthesis by T2 infection of a thymine requiring mutant of Escherichia coli. J Bacteriol. 1954 Jul;68(1):80–88. doi: 10.1128/jb.68.1.80-88.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowlishaw J., Ginoza W. Induction of lambda prophage by nalidixic acid. Virology. 1970 Jun;41(2):244–255. doi: 10.1016/0042-6822(70)90076-0. [DOI] [PubMed] [Google Scholar]
- GALLANT J., SPOTTSWOOD T. ESCAPE SYNTHESIS OF ALKALINE PHOSPHATASE. Biochim Biophys Acta. 1965 May 11;103:109–119. doi: 10.1016/0005-2787(65)90544-7. [DOI] [PubMed] [Google Scholar]
- GALLANT J., SUSKIND S. R. Relationship between thymineless death and ultraviolet inactivation in Escherichia coli. J Bacteriol. 1961 Aug;82:187–194. doi: 10.1128/jb.82.2.187-194.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIUCHI T., HORIUCHI S., MIZUNO D. A possible negative feedback phenomenon controlling formation of alkaline phosphomonoesterase in Escherichia coli. Nature. 1959 May 30;183(4674):1529–1530. doi: 10.1038/1831529b0. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P., Theriot L. Three loci in Escherichia coli K-12 that control the excision of pyrimidine dimers and certain other mutagen products from DNA. Genetics. 1966 Jun;53(6):1119–1136. doi: 10.1093/genetics/53.6.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROODYN D. B., MANDEL H. G. A simple membrane fractionation method for determining the distribution of radioactivity in chemical fractions of Bacillus cereus. Biochim Biophys Acta. 1960 Jun 17;41:80–88. doi: 10.1016/0006-3002(60)90371-1. [DOI] [PubMed] [Google Scholar]
- Walker J. R. Escherichia coli ras locus: its involvement in radiation repair. J Bacteriol. 1969 Sep;99(3):713–719. doi: 10.1128/jb.99.3.713-719.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. R., Pardee A. B. Conditional mutations involving septum formation in Escherichia coli. J Bacteriol. 1967 Jan;93(1):107–114. doi: 10.1128/jb.93.1.107-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



