Abstract
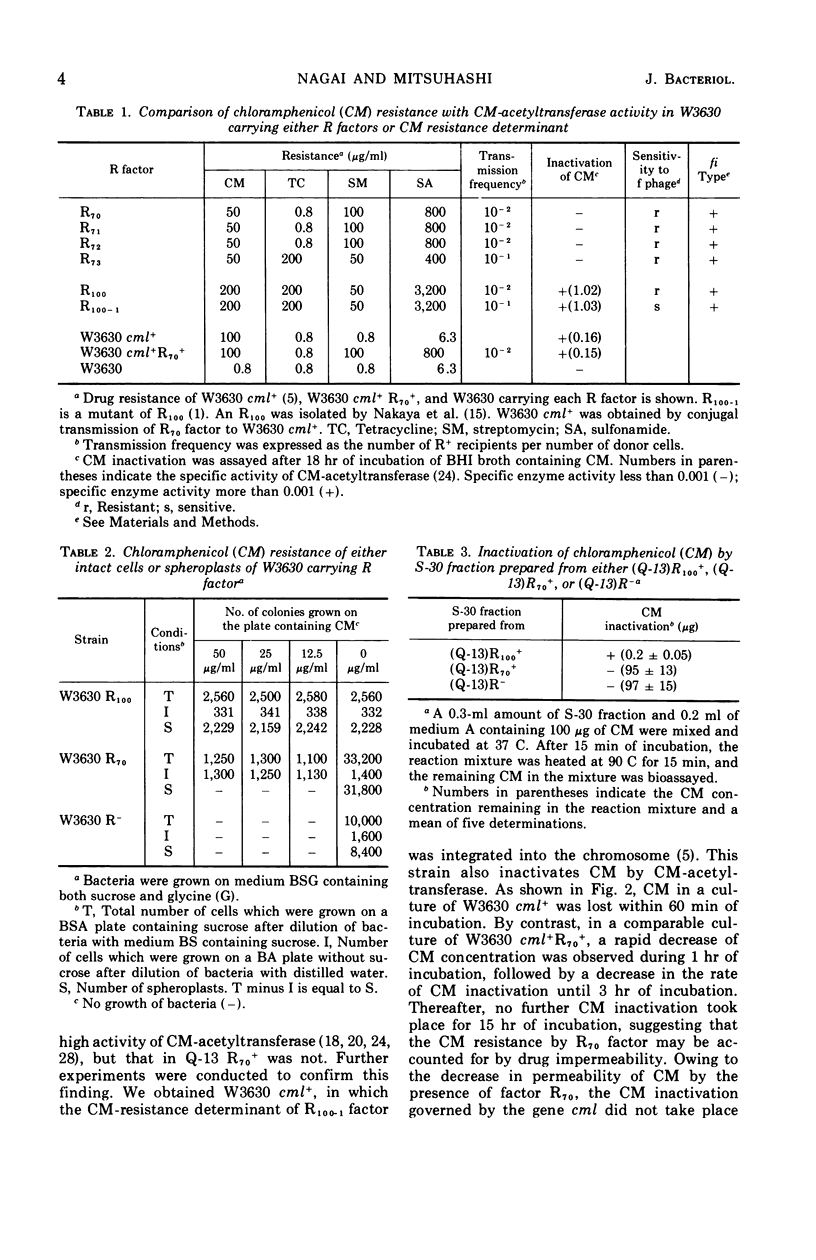
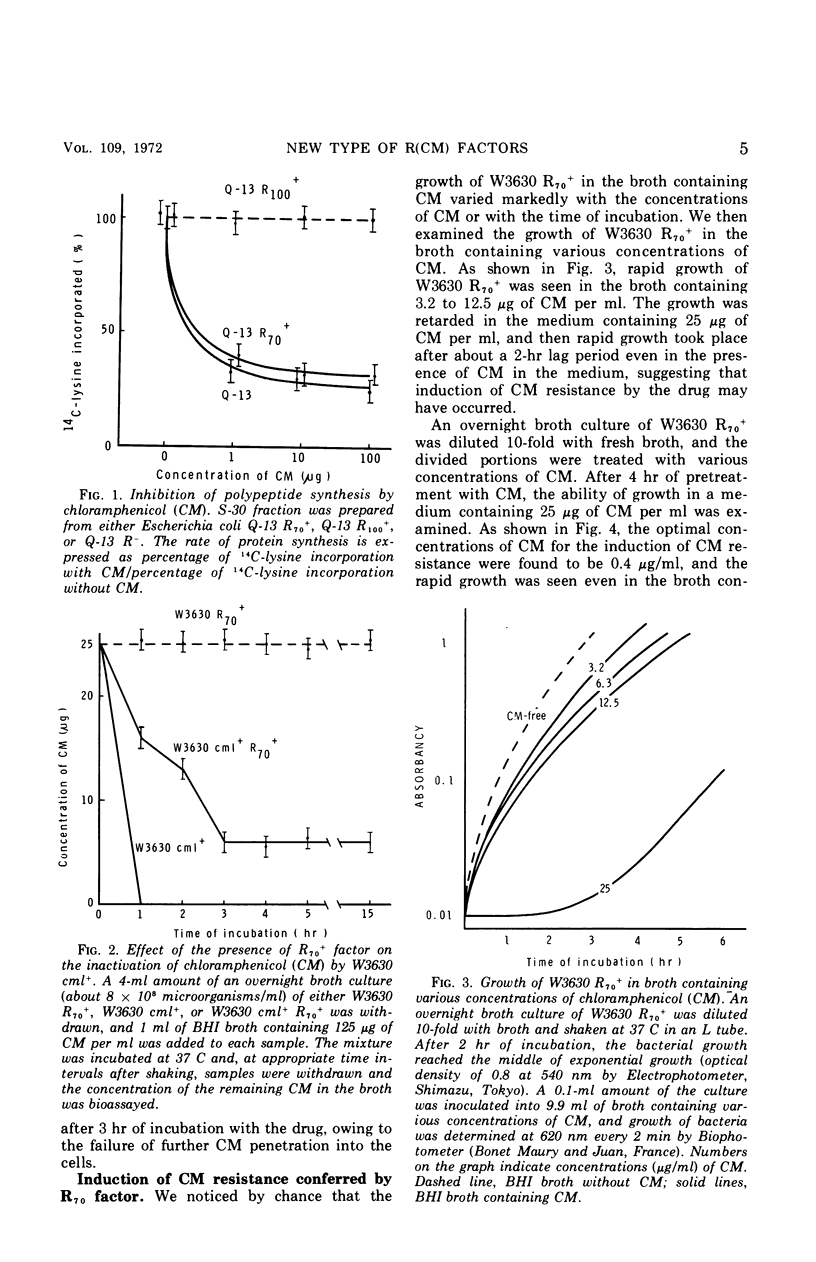
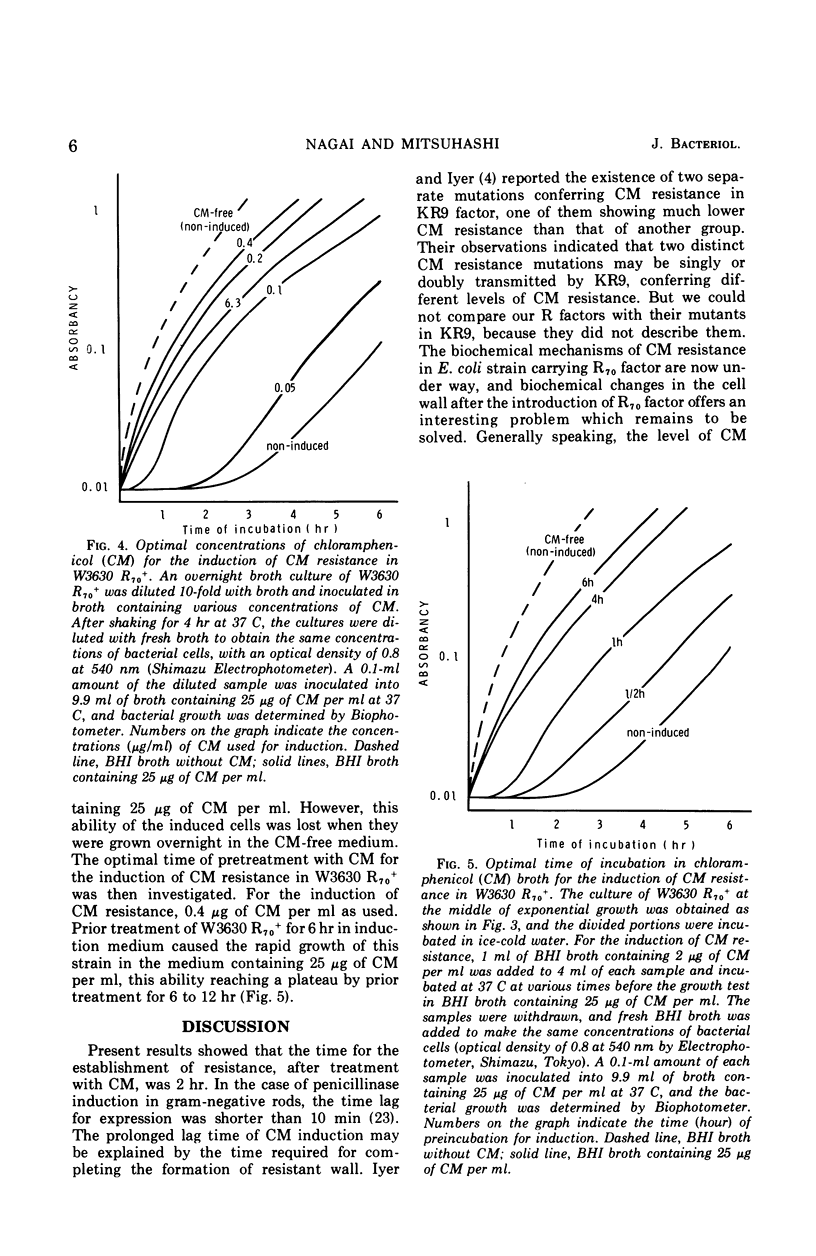
Four R factors conferring chloramphenicol (CM) resistance were isolated from Escherichia coli strains of clinical origin. Strains carrying the factors were found to be incapable of inactivating the drug in the presence of acetyl coenzyme A. E. coli W3630 carrying R70, one of these factors, became sensitive to CM after treatment with glycine, indicating that the spheroplasts of W3630 R70+ were sensitive to the drug and suggesting that the cell membrane is important for CM resistance. The observation that cell-free protein synthesis in W3630 R70+ was inhibited by CM is also compatible with a decrease in permeability. CM resistance in W3630 R70+ appeared to be inducible, because (i) preincubation with subinhibitory concentrations of CM prevented the prolonged lag noted for growth in the presence of 25 μg of CM per ml, and (ii) the preincubation effect was lost after overnight growth in CM-free medium. By contrast, E. coli W3630 cml+, in which the resistance determinant is integrated into the chromosome, was capable of rapid inactivation of CM. E. coli W3630 cml+ R70+, which contains the proposed permeability determinant (episomal) as well as levels of the inactivating enzyme (chromosomal) that are comparable with W3630 cml+, was capable of brief inactivation of CM when inoculated into drug-containing medium. The absence of continued inactivation on more prolonged incubation favors the hypothesis that the R70 factor inhibited further penetration of CM and that this property possesses the characteristics of induction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GARDNER R. S., WAHBA A. J., BASILIO C., MILLER R. S., LENGYEL P., SPEYER J. F. Synthetic polynucleotides and the amino acid code. VII. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2087–2094. doi: 10.1073/pnas.48.12.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARADA K., SUZUKI M., KAMEDA M., MITSUHASHI S. On the drug-resistance of enteric bacteria. 2) Transmission of the drug-resistance among Enterobacteriaceae. Jpn J Exp Med. 1960 Aug;30:289–299. [PubMed] [Google Scholar]
- Iyer R. V., Iyer V. N. Genetic and molecular properties of an infectious antibiotic resistance (R) factor isolated from Klebsiella. J Bacteriol. 1969 Nov;100(2):605–616. doi: 10.1128/jb.100.2.605-616.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAKAMI M., OSAWA N., MITSUHASHI S. Studies on the drug resistance of enteric bacteria. 9. Transmission of the drug-resistance factor between spheroplasts. Jpn J Exp Med. 1961 Aug;31:259–266. [PubMed] [Google Scholar]
- KUSHNER D. J. The basis of chloramphenicol resistance in Pseudomonas fluorescens. Arch Biochem Biophys. 1955 Oct;58(2):347–355. doi: 10.1016/0003-9861(55)90134-x. [DOI] [PubMed] [Google Scholar]
- Kono M., Ogawa K., Mitsuhashi S. Drug resistance of staphylococci. VI. Genetic determinant for chloramphenicol resistance. J Bacteriol. 1968 Mar;95(3):886–892. doi: 10.1128/jb.95.3.886-892.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LOEB T. Isolation of a bacteriophage specific for the F plus and Hfr mating types of Escherichia coli K-12. Science. 1960 Mar 25;131(3404):932–933. doi: 10.1126/science.131.3404.932. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mao J. C. Protein synthesis in a cell-free extract from Staphylococcus aureus. J Bacteriol. 1967 Jul;94(1):80–86. doi: 10.1128/jb.94.1.80-86.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Cooke M. Repressor-minus and operator-constitutive de-repressed mutants of F-like R factors: their effect on chromosomal transfer by HfrC. Genet Res. 1969 Dec;14(3):309–313. doi: 10.1017/s0016672300002123. [DOI] [PubMed] [Google Scholar]
- NAKAYA R., NAKAMURA A., MURATA Y. Resistance transfer agents in Shigella. Biochem Biophys Res Commun. 1960 Dec;3:654–659. doi: 10.1016/0006-291x(60)90081-4. [DOI] [PubMed] [Google Scholar]
- OKAMOTO S., MIZUNO D. Inhibition by chloramphenicol of protein synthesis in the cell-free system of a chloramphenicol-resistant strain of Escherichia coli. Nature. 1962 Sep 8;195:1022–1023. doi: 10.1038/1951022a0. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y., Mise K., Nakaya R. Occurrence of chloramphenicol-acetylating enzymes in various gram-negative bacilli. J Bacteriol. 1967 Nov;94(5):1616–1622. doi: 10.1128/jb.94.5.1616-1622.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaka E., Teraoka H., Tamaki M., Tanaka K., Osawa S. Ribosomes from erythromycin-resistant mutants of Escherichia coli Q13. J Mol Biol. 1970 Mar;48(3):499–510. doi: 10.1016/0022-2836(70)90061-6. [DOI] [PubMed] [Google Scholar]
- RAMSEY H. H. Protein synthesis as a basis for chloramphenicol-resistance in Staphylococcus aureus. Nature. 1958 Aug 30;182(4635):602–603. doi: 10.1038/182602a0. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. Characteristics of some single-step mutants to chloramphenicol resistance in Escherichia coli K12 and their interactions with R-factor genes. Genet Res. 1966 Apr;7(2):281–286. doi: 10.1017/s0016672300009708. [DOI] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M., Saito T., Mitsuhashi S. Macrolide resistance in Staphylococcus aureus. Relation between spiramycin-binding to ribosome and inhibition of polypeptide synthesis in a heat inducible-resistant mutant. Jpn J Microbiol. 1970 Mar;14(2):155–162. [PubMed] [Google Scholar]
- Sompolinsky D., Samra Z. Mechanism of high-level resistance to chloramphenicol in different Escherichia coli variants. J Gen Microbiol. 1968 Jan;50(1):55–66. doi: 10.1099/00221287-50-1-55. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S., Kono M. Basis of chloramphenicol resistance in naturally isolated resistant staphylococci. J Bacteriol. 1966 Sep;92(3):798–799. doi: 10.1128/jb.92.3.798-799.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Okamoto S. The enzymatic acetylation of chloramphenicol by the multiple drug-resistant Escherichia coli carrying R factor. J Biol Chem. 1967 Oct 25;242(20):4722–4730. [PubMed] [Google Scholar]



