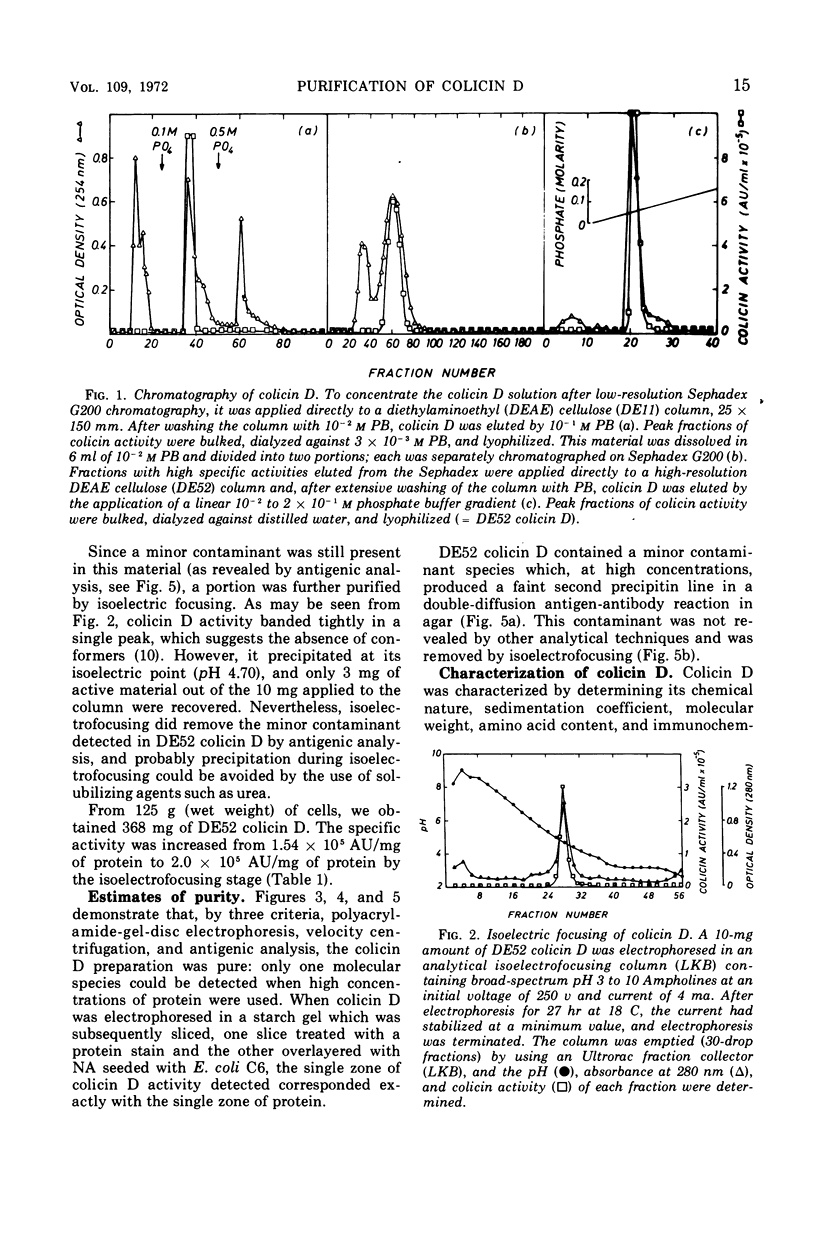
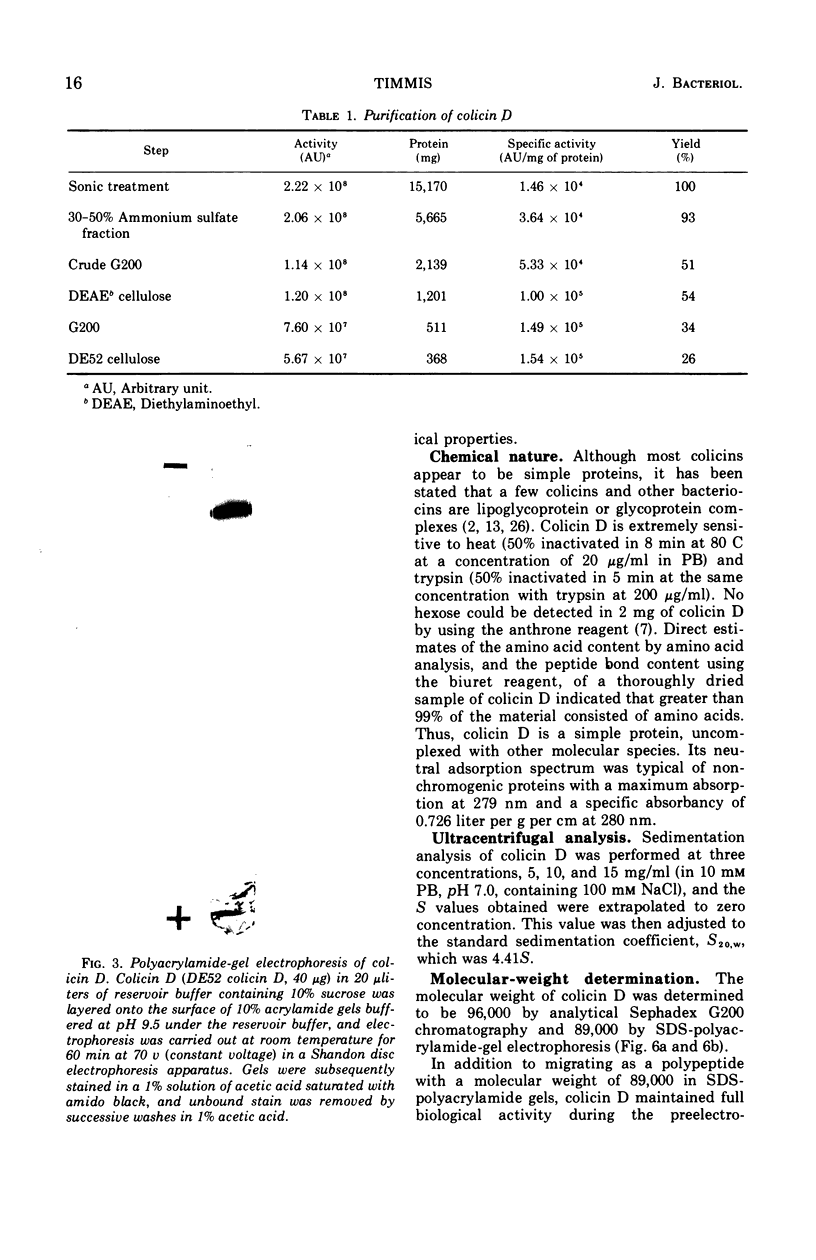
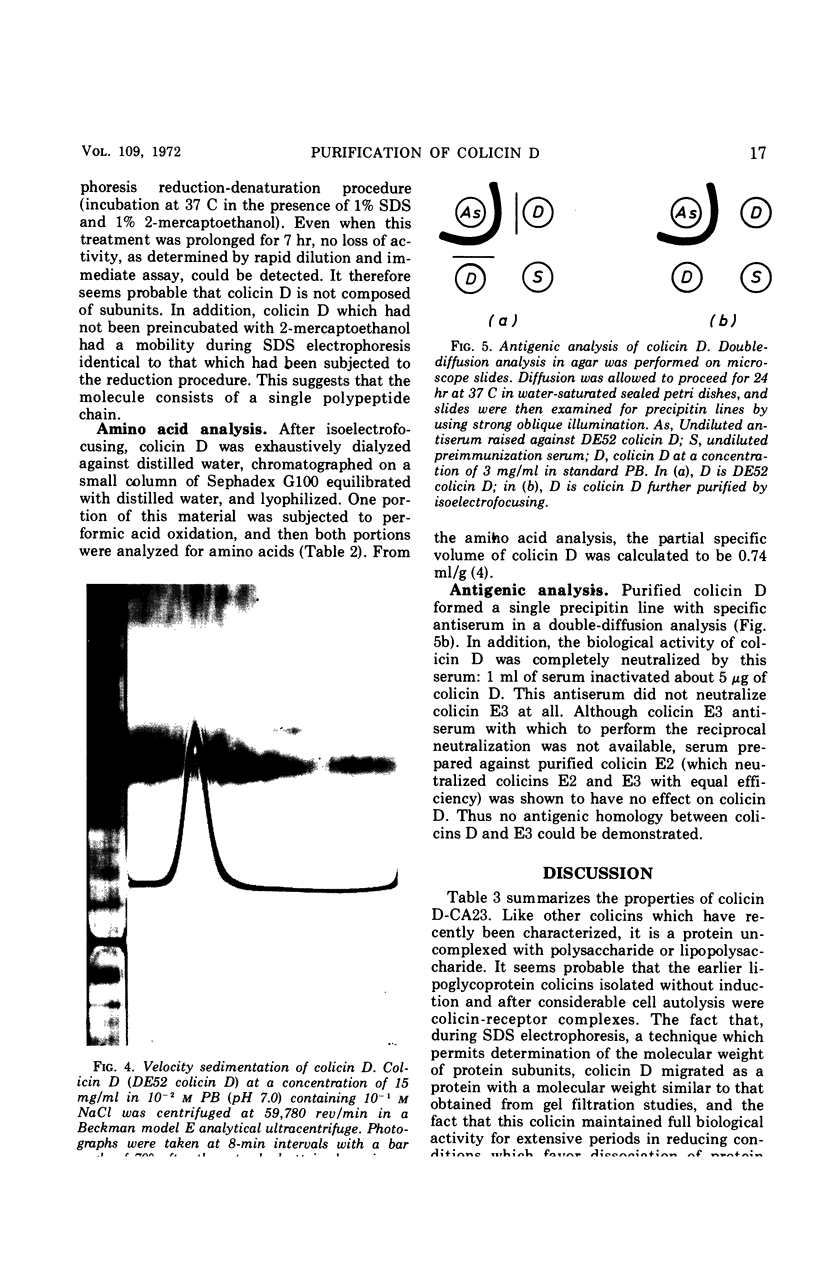
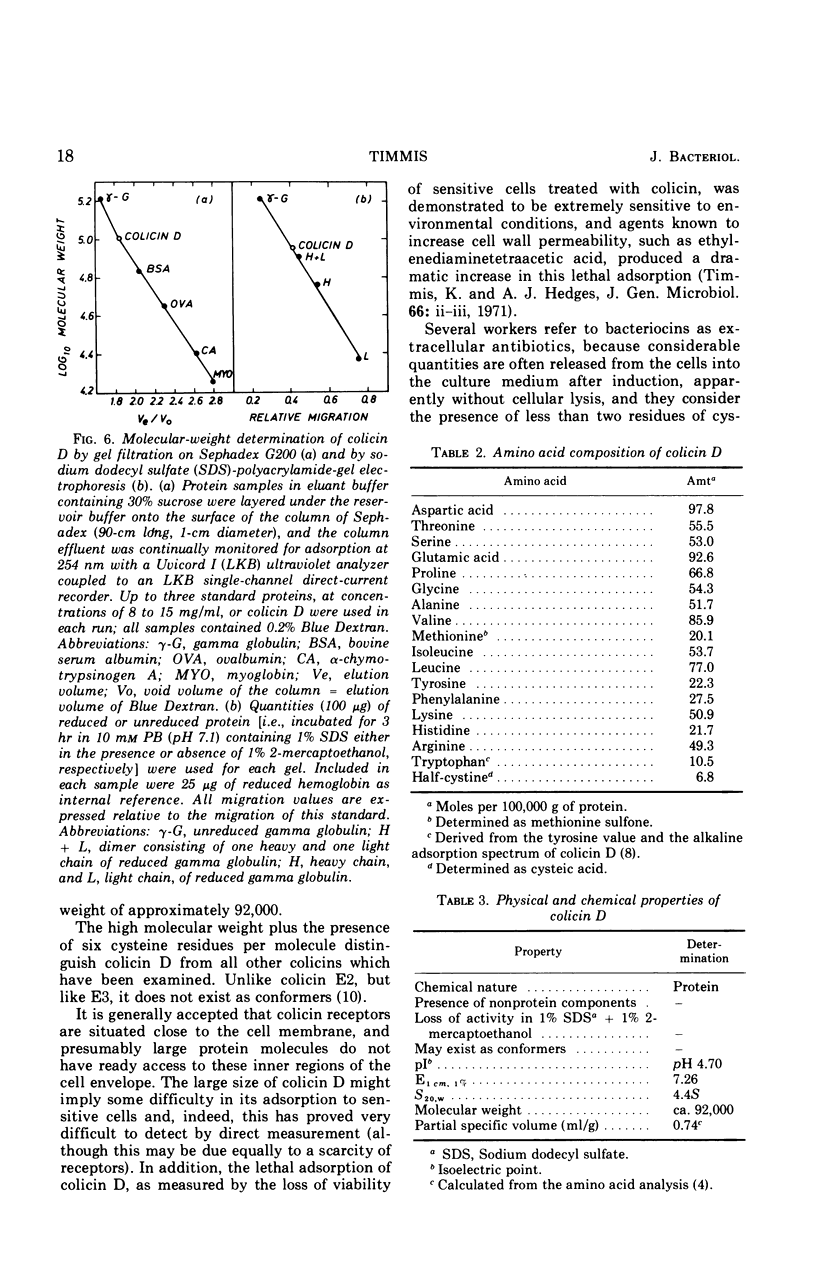
Abstract
Colicin D-CA23, obtained by sonic treatment of mitomycin C-induced cells of Escherichia coli K-12 W1485 (colD), was purified by ammonium sulfate precipitation, gel filtration on Sephadex G200, ion-exchange chromatography on diethylaminoethyl cellulose, and isoelectrofocusing. Polyacrylamide-gel electrophoresis, sedimentation velocity analysis, and antigenic analysis indicated that the preparation was homogeneous. Colicin D is composed entirely of amino acids and hence is a simple protein uncomplexed with lipid or lipopolysaccharide. It contains six residues of cysteine per molecule. The molecular weight of colicin D is approximately 92,000, as determined by sodium dodecyl sulfate-polyacrylamide-gel electrophoresis and gel filtration on Sephadex G200. Its sedimentation coefficient is 4.41S. The behavior of colicin D in solutions of sodium dodecyl sulfate and 2-mercaptoethanol indicates that it does not consist of subunits and exists as a single polypeptide chain. Its high molecular weight and presence of six cysteine residues per molecule distinguish colicin D from all colicins previously described. Although colicins D and E3 have similar modes of action, their gross molecular properties are entirely different.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya P., Wendt L., Whitney E., Silver S. Colicin-tolerant mutants of Escherichia coli: resistance of membranes to colicin E1. Science. 1970 May 22;168(3934):998–1000. doi: 10.1126/science.168.3934.998. [DOI] [PubMed] [Google Scholar]
- COHEN G. H., JOHNSTONE D. B. Acid production by Azotobacter vinelandii. Nature. 1963 Apr 13;198:211–211. doi: 10.1038/198211a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- HINSDILL R. D., GOEBEL W. F. THE CHEMICAL NATURE OF BACTERIOCINS. Ann Inst Pasteur (Paris) 1964 Nov;107:SUPPL–SUPPL:66. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- HUTTON J. J., GOEBEL W. F. Colicine V. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1498–1500. doi: 10.1073/pnas.47.9.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Konisky J., Nomura M. Interaction of colicins with bacterial cells. II. Specific alteration of Escherichia coli ribosomes induced by colicin E3 in vivo. J Mol Biol. 1967 Jun 14;26(2):181–195. doi: 10.1016/0022-2836(67)90290-2. [DOI] [PubMed] [Google Scholar]
- Konisky J., Richards F. M. Characterization of colicin Ia and colicin Ib. Purification and some physical properties. J Biol Chem. 1970 Jun 10;245(11):2972–2978. [PubMed] [Google Scholar]
- Kunugita K., Matsuhashi M. Purification and properties of colicin K. J Bacteriol. 1970 Nov;104(2):1017–1019. doi: 10.1128/jb.104.2.1017-1019.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marotel-Schirmann J., Barbu E. Quelques données sur les récepteurs des colicines. C R Acad Sci Hebd Seances Acad Sci D. 1969 Aug 25;269(8):866–869. [PubMed] [Google Scholar]
- Meynell E., Meynell G. G., Datta N. Phylogenetic relationships of drug-resistance factors and other transmissible bacterial plasmids. Bacteriol Rev. 1968 Mar;32(1):55–83. doi: 10.1128/br.32.1.55-83.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smit J. A., de Klerk H. C., Coetzee J. N. Properties of a Proteus morganii bacteriocin. J Gen Microbiol. 1968 Nov;54(1):67–75. doi: 10.1099/00221287-54-1-67. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]