Abstract
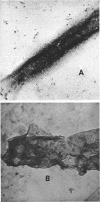
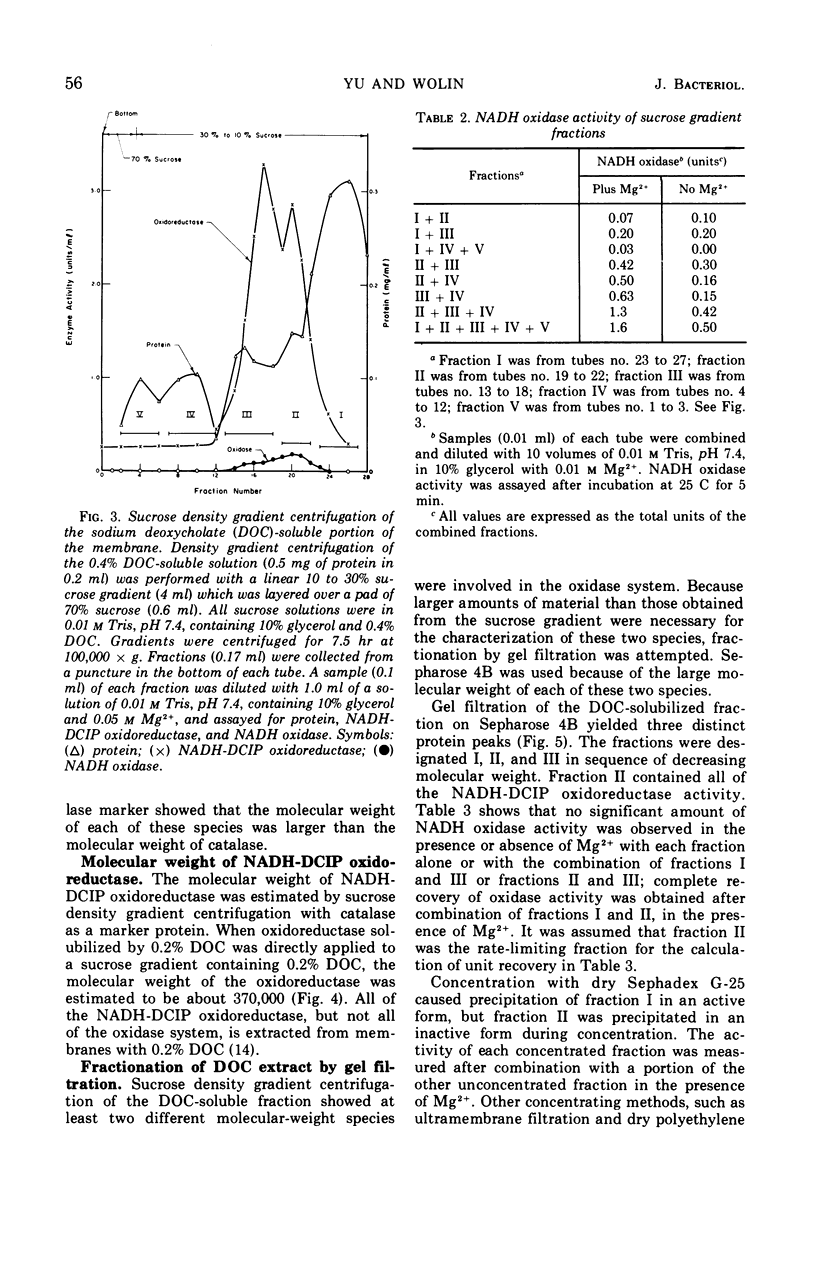
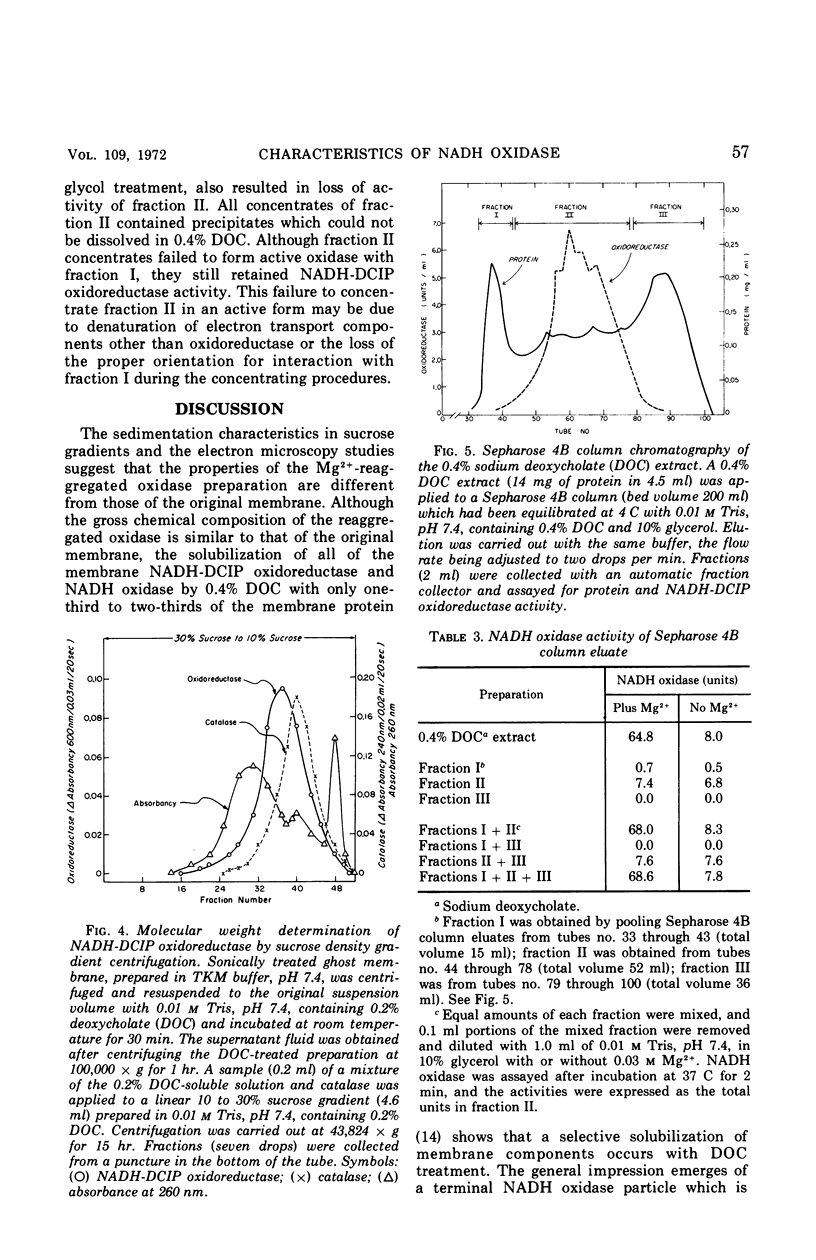
The inactive components of the nicotinamide adenine dinucleotide (reduced form) (NADH) oxidase present in the 0.4% deoxycholate-soluble fraction obtained from Bacillus megaterium KM membranes were reaggregated into active NADH oxidase by dilution in the presence of Mg2+. The reaggregated oxidase was different from the original membrane with respect to sedimentation behavior in a sucrose gradient and morphological appearance. The deoxycholate-insoluble portion of the membrane had membrane-like structure whereas the reaggregated oxidase appeared to be a filamentous aggregate of small particles. The reaggregated oxidase and the deoxycholate-insoluble membrane residue were similar to the original membrane with respect to total protein and total lipid content. The inactive components of the NADH oxidase system exist in deoxycholate as two molecular species which were separable by sucrose density gradient centrifugation or gel filtration in deoxycholate-containing solutions. Both components and dilution in the presence of Mg2+ were necessary for restoration of oxidase activity. The smaller-molecular-weight component contained all of the NADH-2,6-dichlorophenolindophenol oxidoreductase activity of the original membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Spectrophotometric method for the determination of free pentose and pentose in nucleotides. J Biol Chem. 1949 Nov;181(1):379–392. [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Divalent cation activation of deoxycholate-solubilized and -inactivated membrane reduced nicotinamide adenine dinucleotide oxidase of Bacillus megaterium KM. J Bacteriol. 1970 Apr;102(1):172–177. doi: 10.1128/jb.102.1.172-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Masking of Bacillus megaterium KM membrane reduced nicotinamide adenine dinucleotide oxidase and solubilization studies. J Bacteriol. 1970 Apr;102(1):161–171. doi: 10.1128/jb.102.1.161-171.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Niederman R. A., Wolin M. J. Chemical constituents and hydrogenase binding in cell envelopes of Vibrio succinogenes. J Bacteriol. 1969 Apr;98(1):160–166. doi: 10.1128/jb.98.1.160-166.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Salton M. R., Freer J. H., Ellar D. J. Electron transport components localized in a lipid-depleted sheet isolated from Micrococcus lysodeikticus membranes by deoxycholate extraction. Biochem Biophys Res Commun. 1968 Dec 30;33(6):909–915. doi: 10.1016/0006-291x(68)90398-7. [DOI] [PubMed] [Google Scholar]
- Salton M. R. Structure and function of bacterial cell membranes. Annu Rev Microbiol. 1967;21:417–442. doi: 10.1146/annurev.mi.21.100167.002221. [DOI] [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Factors affecting deoxycholate inactivation and Mg++ reactivation of Bacillus megaterium KM membrane nicotinamide adenine dinucleotide (reduced form) oxidase. J Bacteriol. 1970 Aug;103(2):467–474. doi: 10.1128/jb.103.2.467-474.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Separation of the primary dehydrogenase from the cytochromes of the nicotinamide adenine dinucleotide (reduced form) oxidase of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):59–68. doi: 10.1128/jb.109.1.59-68.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]