Abstract
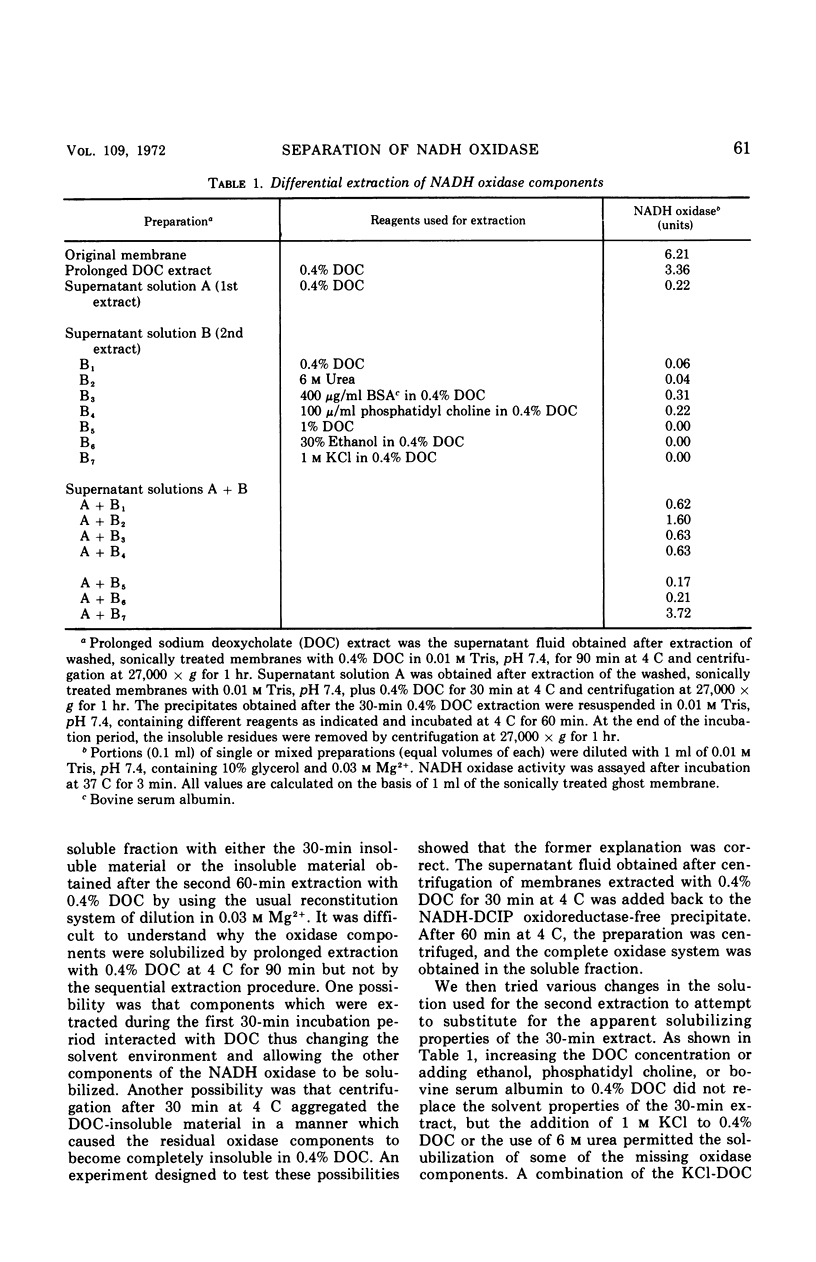
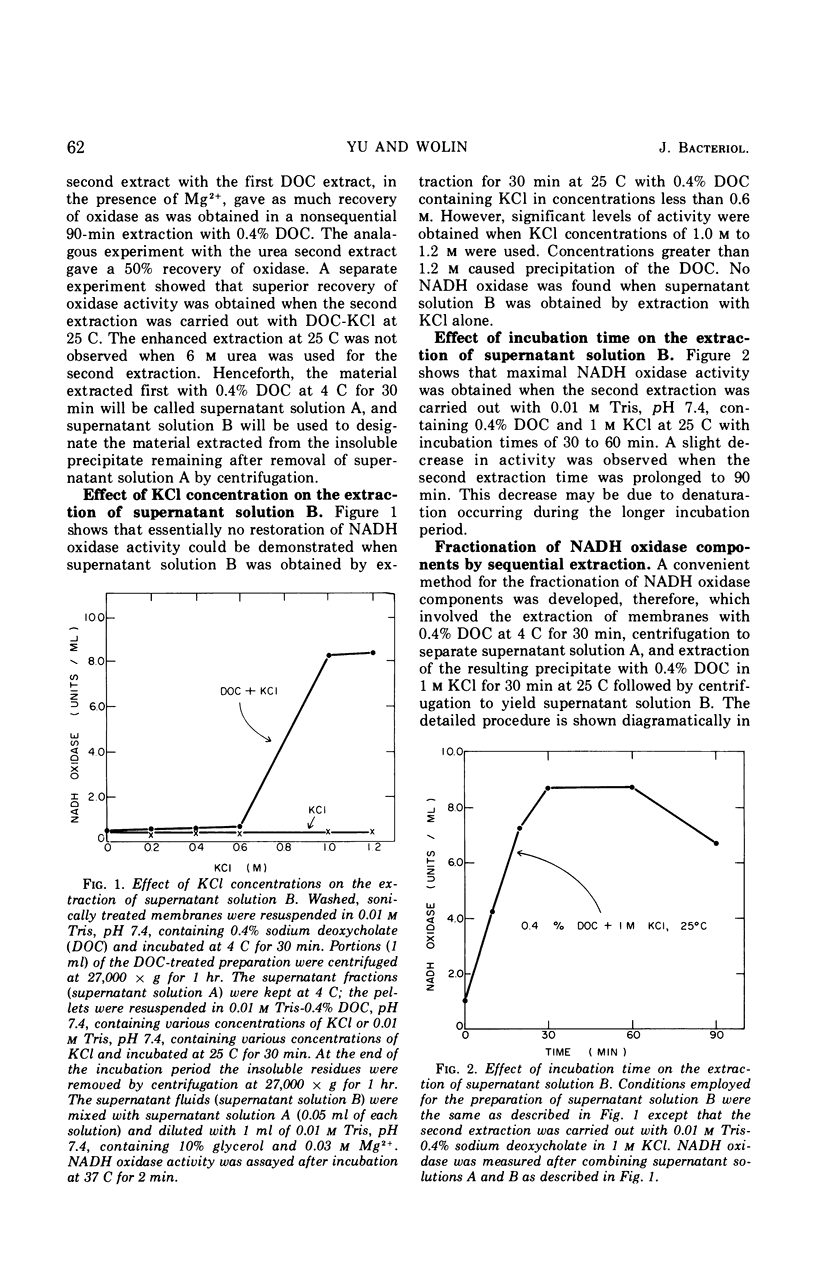
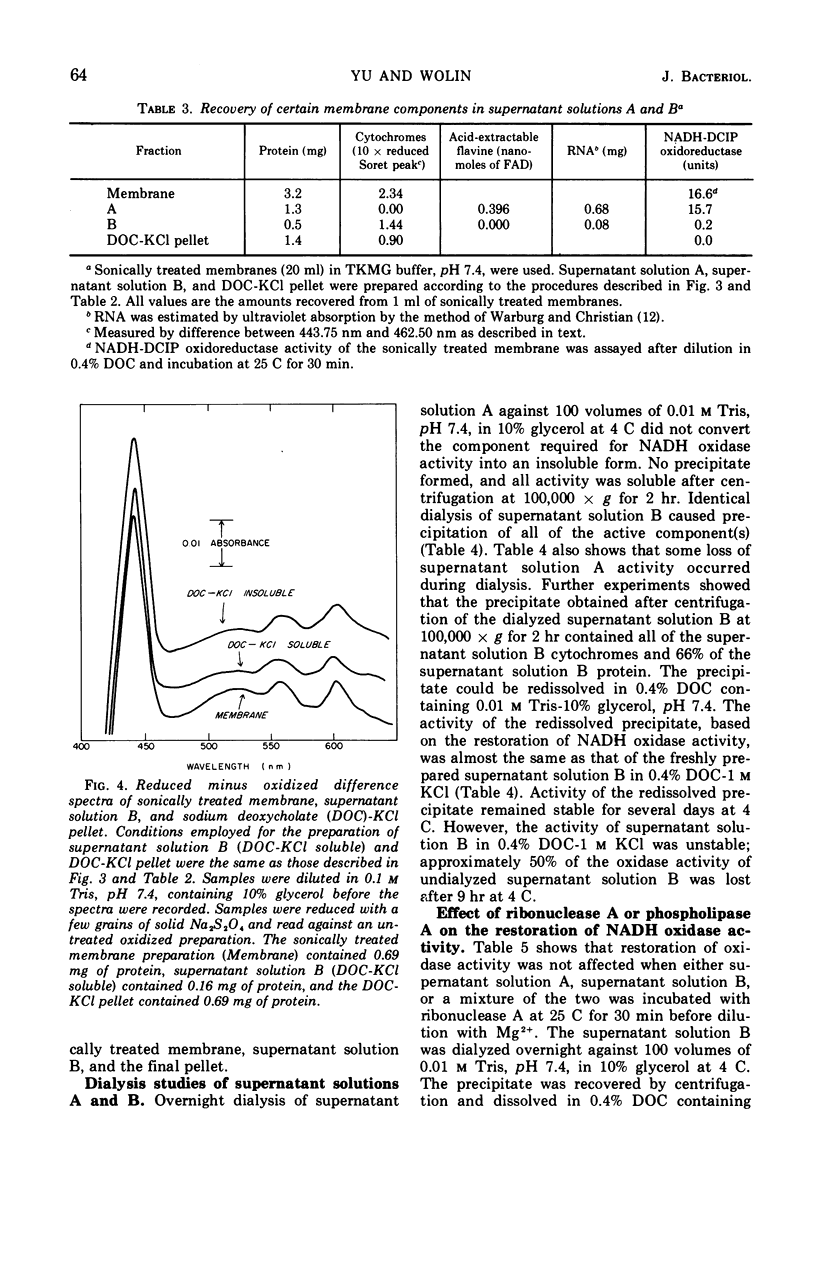
A selective extraction procedure was developed for sequentially extracting a fraction containing the primary dehydrogenase and a fraction containing the cytochromes of the nicotinamide adenine dinucleotide (reduced form) (NADH) oxidase of Bacillus megaterium KM membranes. The primary dehydrogenase (NADH-2,6-dichlorophenolindophenol oxidoreductase) activity was extracted from sonically treated membranes with 0.4% sodium deoxycholate for 30 min at 4 C. The insoluble residue was extracted with 0.4% sodium deoxycholate in 1 m KCl for 30 min at 25 C. A combination of the two extracts and dilution in Mg2+ gave good recovery of the original membrane NADH oxidase activity. The primary dehydrogenase fraction contained 41% of the membrane protein, no cytochromes, flavine adenine dinucleotide as the sole acid-extractable flavine, and most of the membrane ribonucleic acid (RNA). The cytochrome-containing fraction had 16% of the membrane protein, 61% of the membrane cytochrome with the same relative amounts of cytochromes a and b as the original membrane, no acid-extractable flavine, little RNA, and no oxidoreductase activity. The oxidoreductase fraction remained soluble after removal of deoxycholate whereas the cytochrome fraction became insoluble after removal of deoxycholate-KCl, but the precipitated fraction could be redissolved in 0.4% sodium deoxycholate. Treatment of both fractions with ribonuclease to destroy all of the RNA present did not affect the ability of the fractions to recombine into a functional oxidase unit. Treatment of either fraction with phospholipase A prevented restoration of a functional oxidase when the oxidoreductase and cytochrome fractions were treated in solution, but no affect on restoration of oxidase was observed when the phospholipase A treatment was carried out with the soluble oxidoreductase fraction and the insoluble cytochrome fraction.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Divalent cation activation of deoxycholate-solubilized and -inactivated membrane reduced nicotinamide adenine dinucleotide oxidase of Bacillus megaterium KM. J Bacteriol. 1970 Apr;102(1):172–177. doi: 10.1128/jb.102.1.172-177.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. C., Yu L., Wolin M. J. Masking of Bacillus megaterium KM membrane reduced nicotinamide adenine dinucleotide oxidase and solubilization studies. J Bacteriol. 1970 Apr;102(1):161–171. doi: 10.1128/jb.102.1.161-171.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOWLER L. R., RICHARDSON S. H., HATEFI Y. A rapid method for the preparation of highly purified cytochrome oxidase. Biochim Biophys Acta. 1962 Oct 8;64:170–173. doi: 10.1016/0006-3002(62)90770-9. [DOI] [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., FOWLER L. R., GRIFFITHS D. E. Studies on the electron transfer system. XLII. Reconstitution of the electron transfer system. J Biol Chem. 1962 Aug;237:2661–2669. [PubMed] [Google Scholar]
- HATEFI Y., HAAVIK A. G., GRIFFITHS D. E. Studies on the electron transfer system. XLI. Reduced coenzyme Q (QH2)-cytochrome c reductase. J Biol Chem. 1962 May;237:1681–1685. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Isolation and enzymatic properties of the mitochondrial reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1969 May 10;244(9):2350–2357. [PubMed] [Google Scholar]
- Hatefi Y., Stempel K. E. Resolution of complex I (DPNH-coenzyme Q reductase) of the mitochondrial electron transfer system. Biochem Biophys Res Commun. 1967 Feb 8;26(3):301–308. doi: 10.1016/0006-291x(67)90122-2. [DOI] [PubMed] [Google Scholar]
- RAO N. A., FELTON S. P., HUENNEKENS F. M., MACKLER B. Flavin mononucleotide: the coenzyme of reduced diphosphopyridine nucleotide dehydrogenase. J Biol Chem. 1963 Jan;238:449–455. [PubMed] [Google Scholar]
- Tzagoloff A., MacLennan D. H., McConnell D. G., Green D. E. Studies on the electron transfer system. 68. Formation of membranes as the basis of the reconstitution of the mitochondrial electron transfer system. J Biol Chem. 1967 May 10;242(9):2051–2061. [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Chemical and physical characteristics of the deoxycholate-soluble and magnesium-reaggregated membrane nicotinamide adenine dinucleotide (reduced form) oxidase of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):51–58. doi: 10.1128/jb.109.1.51-58.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Wolin M. J. Factors affecting deoxycholate inactivation and Mg++ reactivation of Bacillus megaterium KM membrane nicotinamide adenine dinucleotide (reduced form) oxidase. J Bacteriol. 1970 Aug;103(2):467–474. doi: 10.1128/jb.103.2.467-474.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



