Abstract
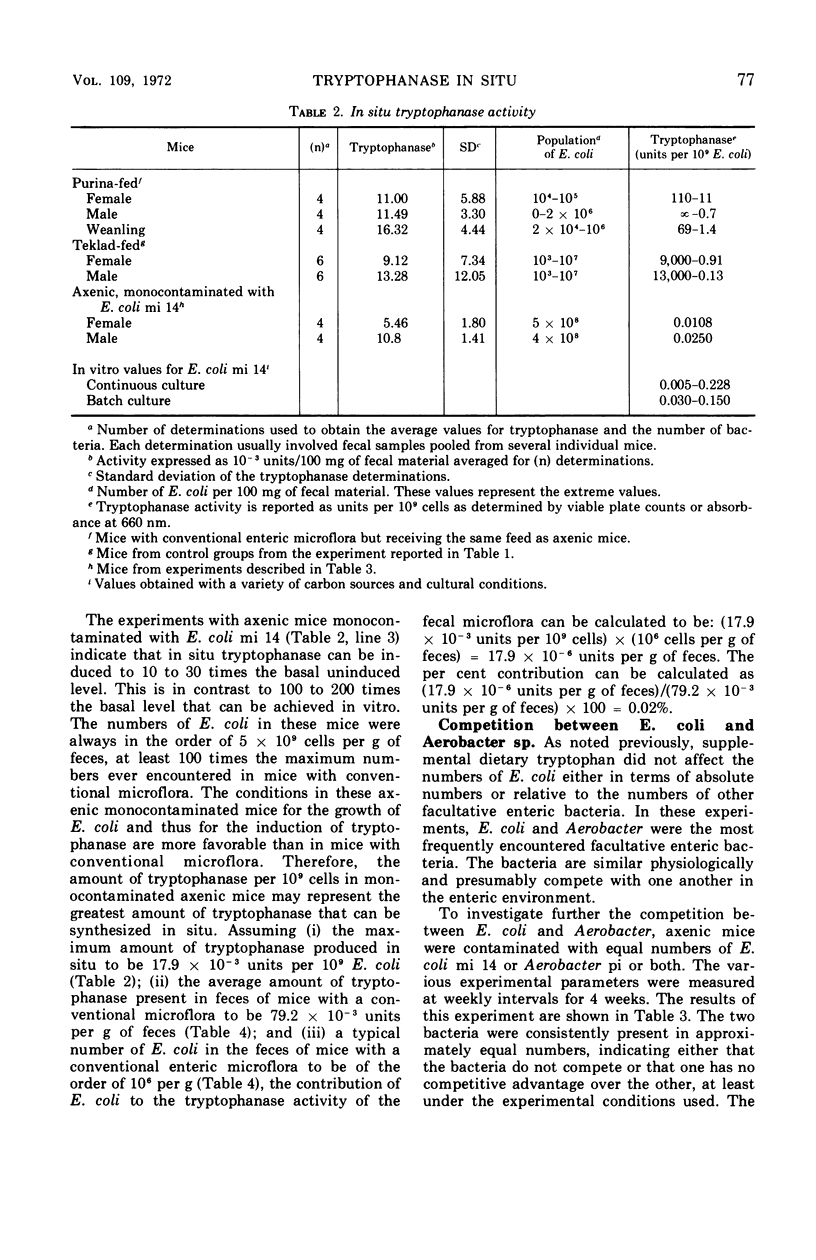
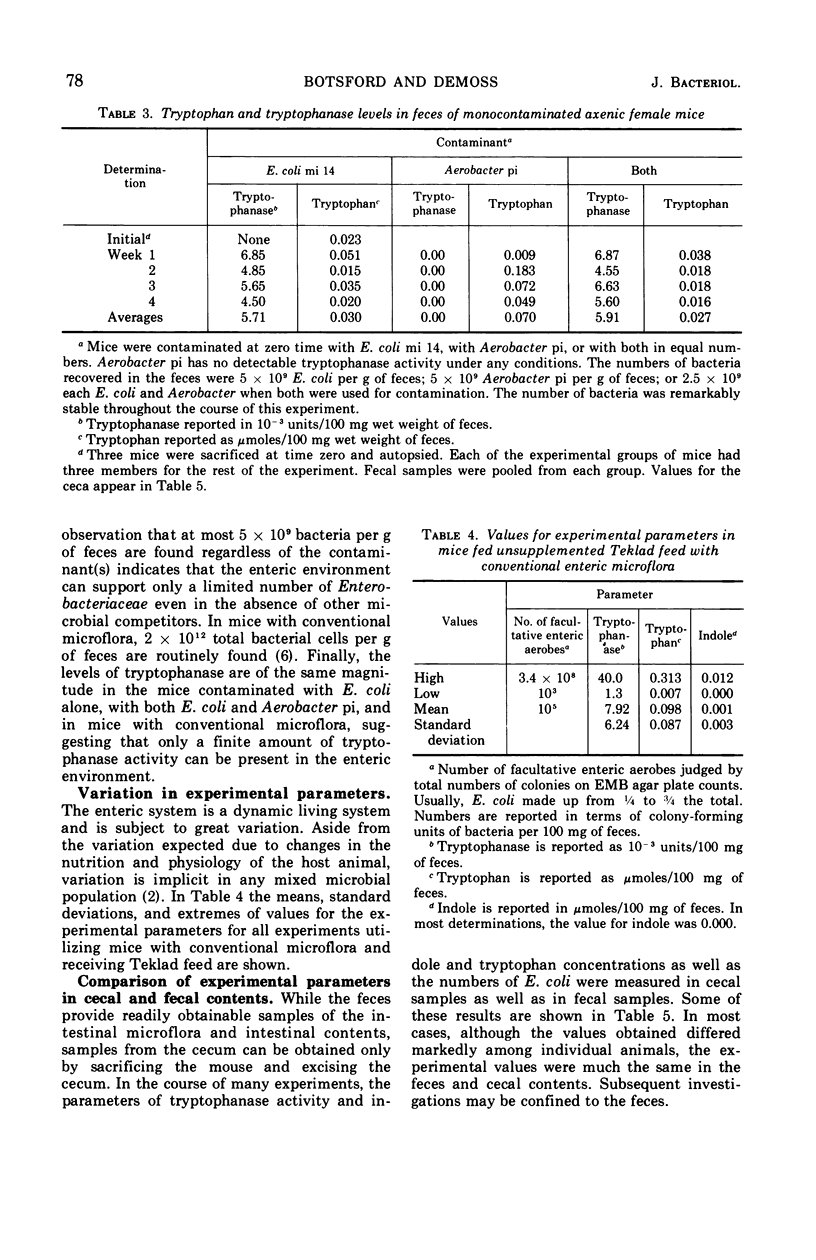
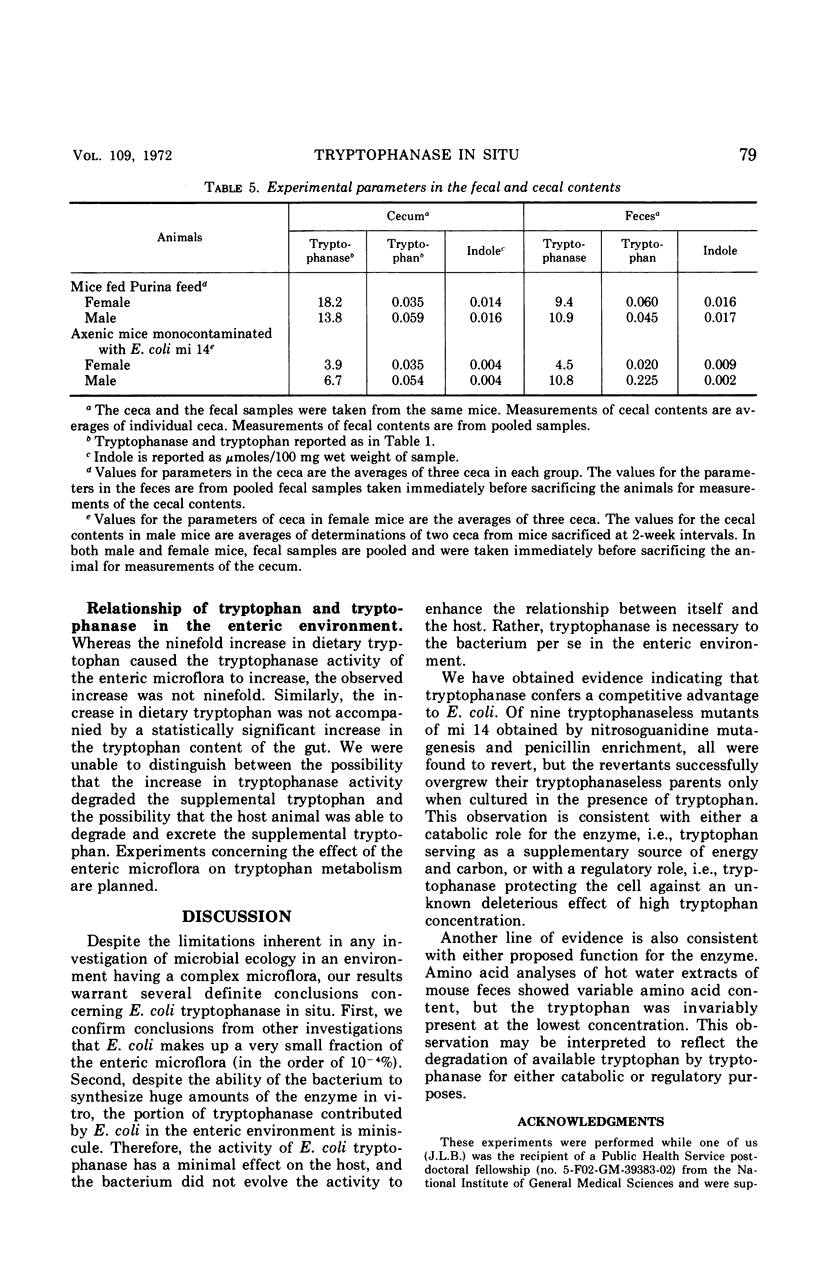
The activity of the enzyme tryptophanase in the enteric environment was investigated to elucidate the significance of the enzyme in the metabolism of Escherichia coli. The tryptophanase activity, tryptophan content, and indole concentration as well as the numbers of E. coli were determined in the intestinal and fecal contents of conventional, germ-free, and monocontaminated axenic laboratory mice. Increasing the tryptophan content of the diet of mice having a conventional microflora increased the tryptophanase activity of the enteric microflora by a factor of almost 2 but did not increase the numbers of E. coli either absolutely or relative to other facultative enteric coliforms. In the enteric environment, E. coli is responsible for very little tryptophanase activity, a fraction calculated to be less than 0.02%. The values for the experimental parameters were much the same in the contents of the cecum and in the fecal material.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., DEMOSS R. D. Properties of tryptophanase from Escherichia coli. Biochim Biophys Acta. 1962 Dec 4;65:233–244. doi: 10.1016/0006-3002(62)91042-9. [DOI] [PubMed] [Google Scholar]
- Botsford J. L., DeMoss R. D. Catabolite repression of tryptophanase in Escherichia coli. J Bacteriol. 1971 Jan;105(1):303–312. doi: 10.1128/jb.105.1.303-312.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bungay H. R., 3rd, Bungay M. L. Microbial interactions in continuous culture. Adv Appl Microbiol. 1968;10:269–290. doi: 10.1016/s0065-2164(08)70194-1. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOS R., SCHAEDLER R. W., COSTELLO R., HOET P. INDIGENOUS, NORMAL, AND AUTOCHTHONOUS FLORA OF THE GASTROINTESTINAL TRACT. J Exp Med. 1965 Jul 1;122:67–76. doi: 10.1084/jem.122.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss R. D., Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969 Apr;98(1):167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANK L. H., DEMOSS R. D. Specific enzymic method for the estimation of L-tryptophan. Arch Biochem Biophys. 1957 Apr;67(2):387–397. doi: 10.1016/0003-9861(57)90293-x. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Kapsimalis B. Estimates of the overall rate of growth of the intestinal microflora of hamsters, guinea pigs, and mice. J Bacteriol. 1967 Jan;93(1):510–512. doi: 10.1128/jb.93.1.510-512.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S. K., DeMoss R. D. Physiological comparison of L-serine dehydratase and tryptophanase from Bacillus alvei. J Bacteriol. 1970 Mar;101(3):813–820. doi: 10.1128/jb.101.3.813-820.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., DeMoss R. D. Physiological role of tryptophanase in control of tryptophan biosynthesis in Bacillus alvei. J Bacteriol. 1966 Feb;91(2):667–672. doi: 10.1128/jb.91.2.667-672.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroute F. Regulation of pyrimidine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1968 Mar;95(3):824–832. doi: 10.1128/jb.95.3.824-832.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Regulation of sugar transport in Neurospora crassa. J Bacteriol. 1971 May;106(2):487–492. doi: 10.1128/jb.106.2.487-492.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]



