Abstract
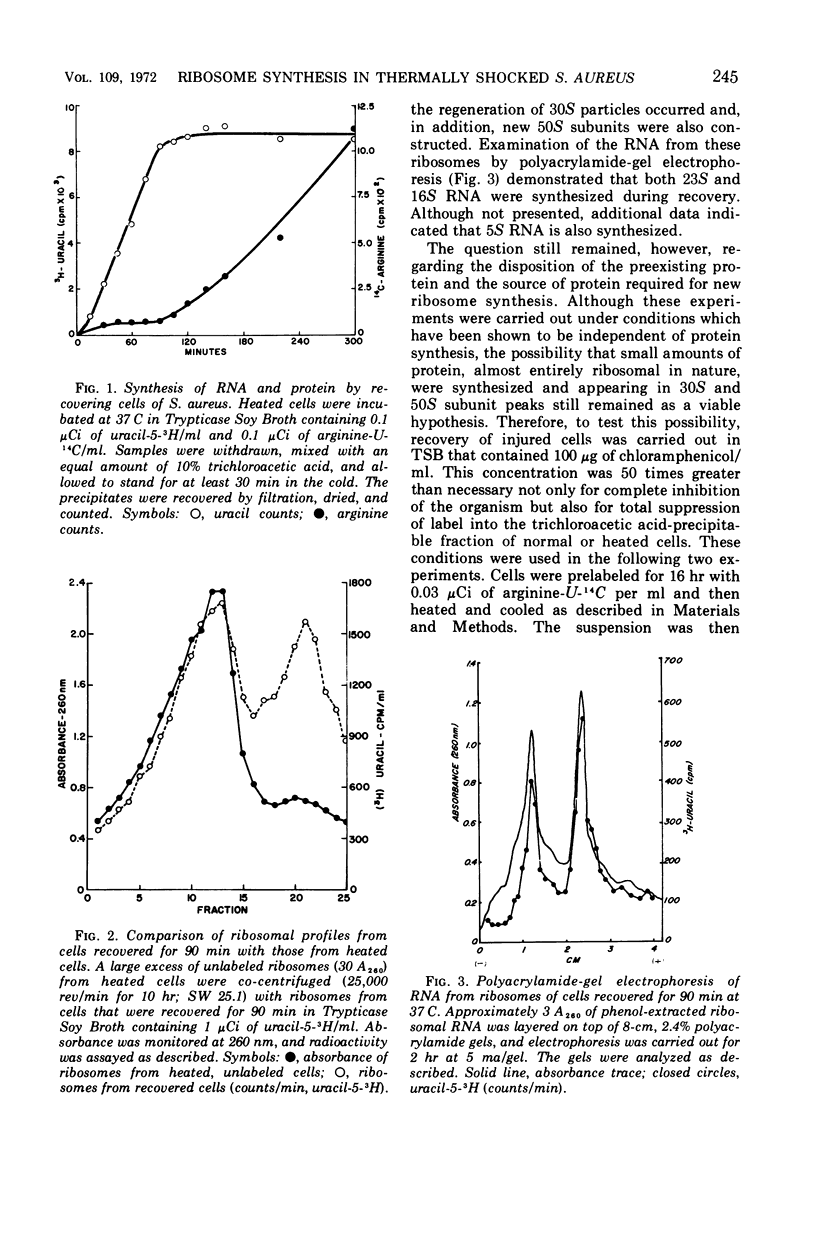
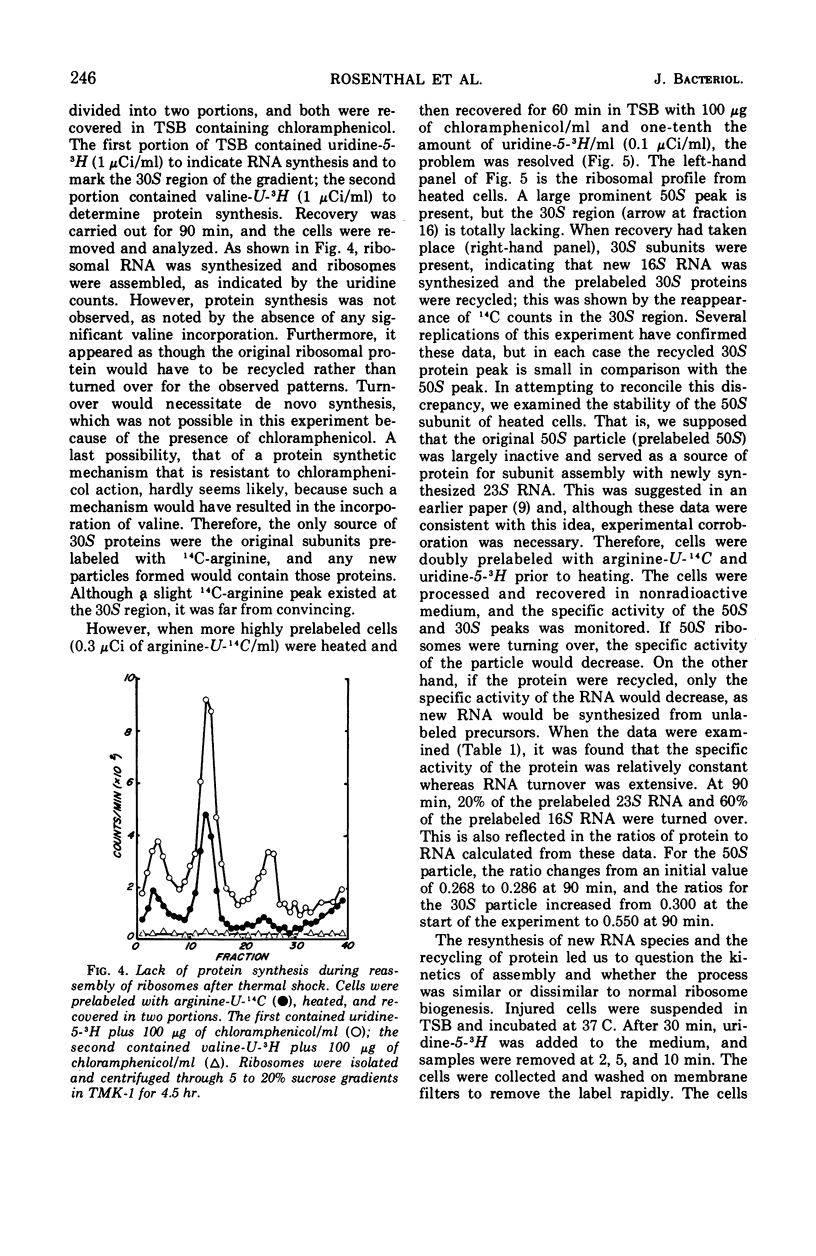
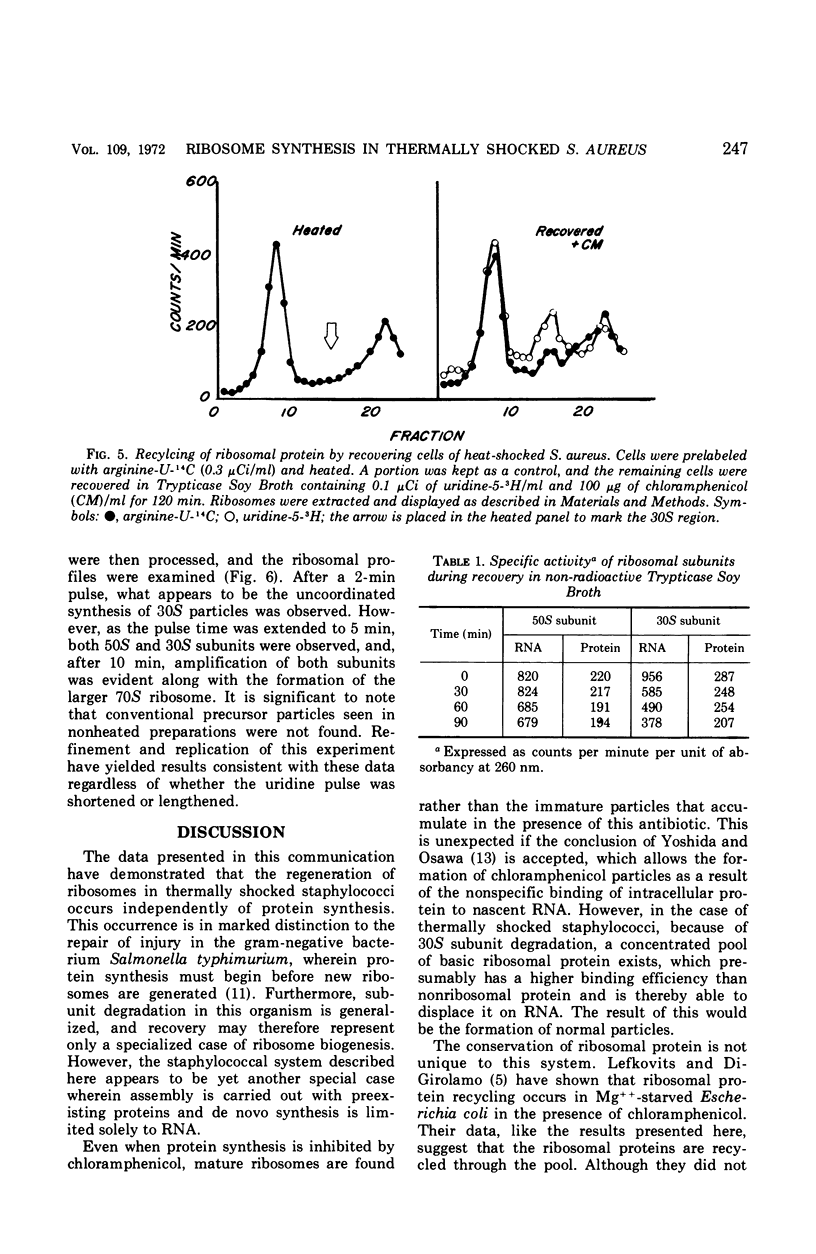
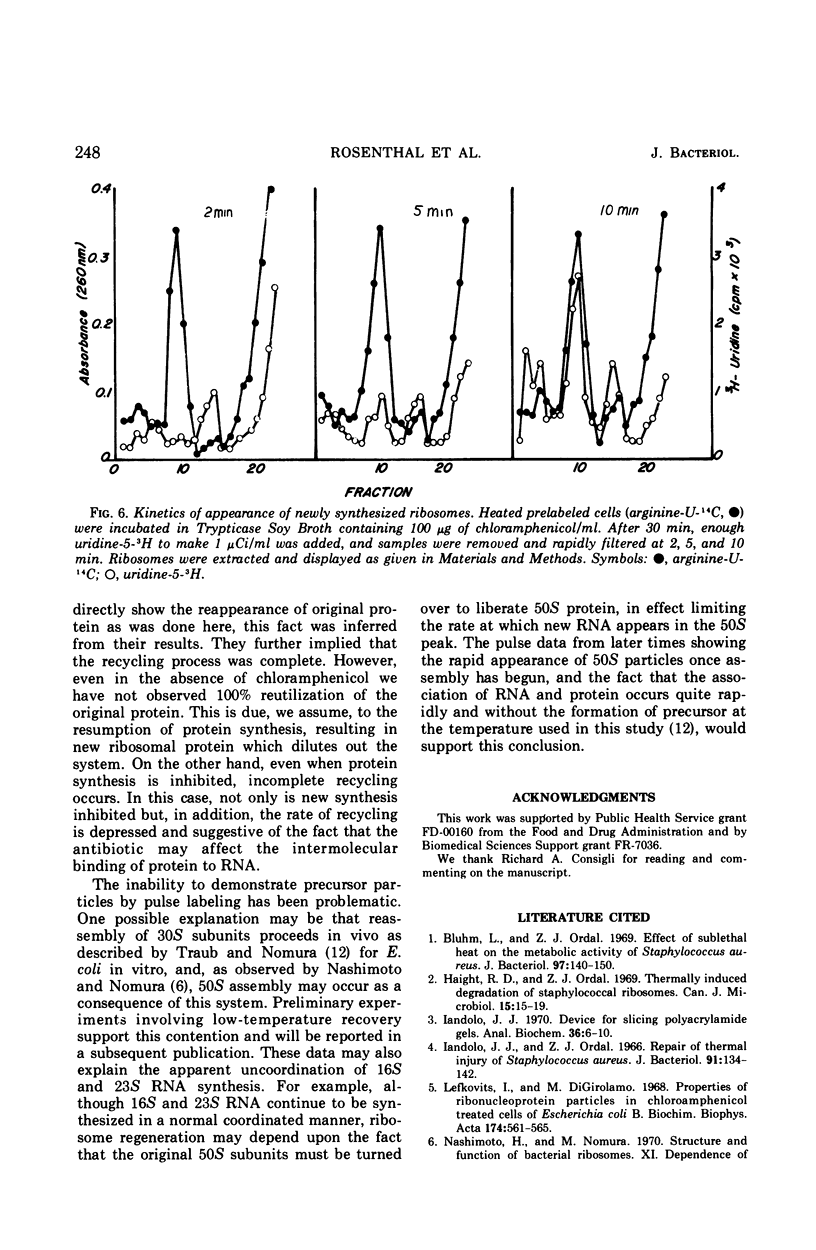
Thermally shocked cells of Staphylococcus aureus rapidly synthesized ribonucleic acid (RNA) during the early stages of recovery. During this period, protein synthesis was not observed and occurred only after RNA had reached a maximum level. Even in the absence of coordinated protein synthesis, a large portion of the RNA appeared in newly synthesized ribosomes. Although the 30S subunit was specifically destroyed by the heating process, both ribosomal particles were reassembled during recovery. The addition of chloramphenicol did not inhibit the formation of the ribosomal subunits, nor was the presence of immature chloramphenicol particles detected. Extended recovery with highly prelabeled cells showed that the original ribosomal proteins present before heating are conserved and recycled. Furthermore, the data indicate that the 50S subunit is turned over and used as a source of protein for new ribosome assembly. Kinetic studies of the assembly process by pulse labeling have not revealed the presence of the normally reported precursor particles. Rather, the data suggest that assembly may occur, in this system, in a manner similar to that reported for in vitro assembly of Escherichia coli subunits.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bluhm L., Ordal Z. J. Effect of sublethal heat on the metabolic activity of Staphylococcus aureus. J Bacteriol. 1969 Jan;97(1):140–150. doi: 10.1128/jb.97.1.140-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haight R. D., Ordal Z. J. Thermally induced degradation of staphylococcal ribosomes. Can J Microbiol. 1969 Jan;15(1):15–19. doi: 10.1139/m69-003. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J. Device for slicing polyacrylamide gels. Anal Biochem. 1970 Jul;36(1):6–10. doi: 10.1016/0003-2697(70)90325-8. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkovits I., Di Girolamo M. Properties of ribonucleoprotein particles in chloramphenicol-treated cells of Escherichia coli B. Biochim Biophys Acta. 1969 Feb 18;174(2):561–565. doi: 10.1016/0005-2787(69)90285-8. [DOI] [PubMed] [Google Scholar]
- PATTERSON M. S., GREENE R. C. MEASUREMENT OF LOW ENERGY BETA-EMITTERS IN AQUEOUS SOLUTION BY LIQUID SCINTILLATION COUNTING OF EMULSIONS. Anal Chem. 1965 Jun;37:854–857. doi: 10.1021/ac60226a017. [DOI] [PubMed] [Google Scholar]
- Pariza M. W., Iandolo J. J. Coagulase production by injured Staphylococcus aureus MF-31 during recovery. Appl Microbiol. 1969 Jun;17(6):836–838. doi: 10.1128/am.17.6.836-838.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal L. J., Iandolo J. J. Thermally induced intracellular alteration of ribosomal ribonucleic acid. J Bacteriol. 1970 Sep;103(3):833–835. doi: 10.1128/jb.103.3.833-835.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin S. J., Ordal Z. J. Regeneration of ribosomes and ribosomal ribonucleic acid during repair of thermal injury to Staphylococcus. J Bacteriol. 1967 Oct;94(4):1082–1087. doi: 10.1128/jb.94.4.1082-1087.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlins R. I., Ordal Z. J. Requirements of Salmonella typhimurium for recovery from thermal injury. J Bacteriol. 1971 Feb;105(2):512–518. doi: 10.1128/jb.105.2.512-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Studies on the assembly of ribosomes in vitro. Cold Spring Harb Symp Quant Biol. 1969;34:63–67. doi: 10.1101/sqb.1969.034.01.010. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Osawa S. Origin of the protein component of chlormaphenicaol particles in Escherichia coli. J Mol Biol. 1968 May 14;33(3):559–569. doi: 10.1016/0022-2836(68)90306-9. [DOI] [PubMed] [Google Scholar]



