Abstract
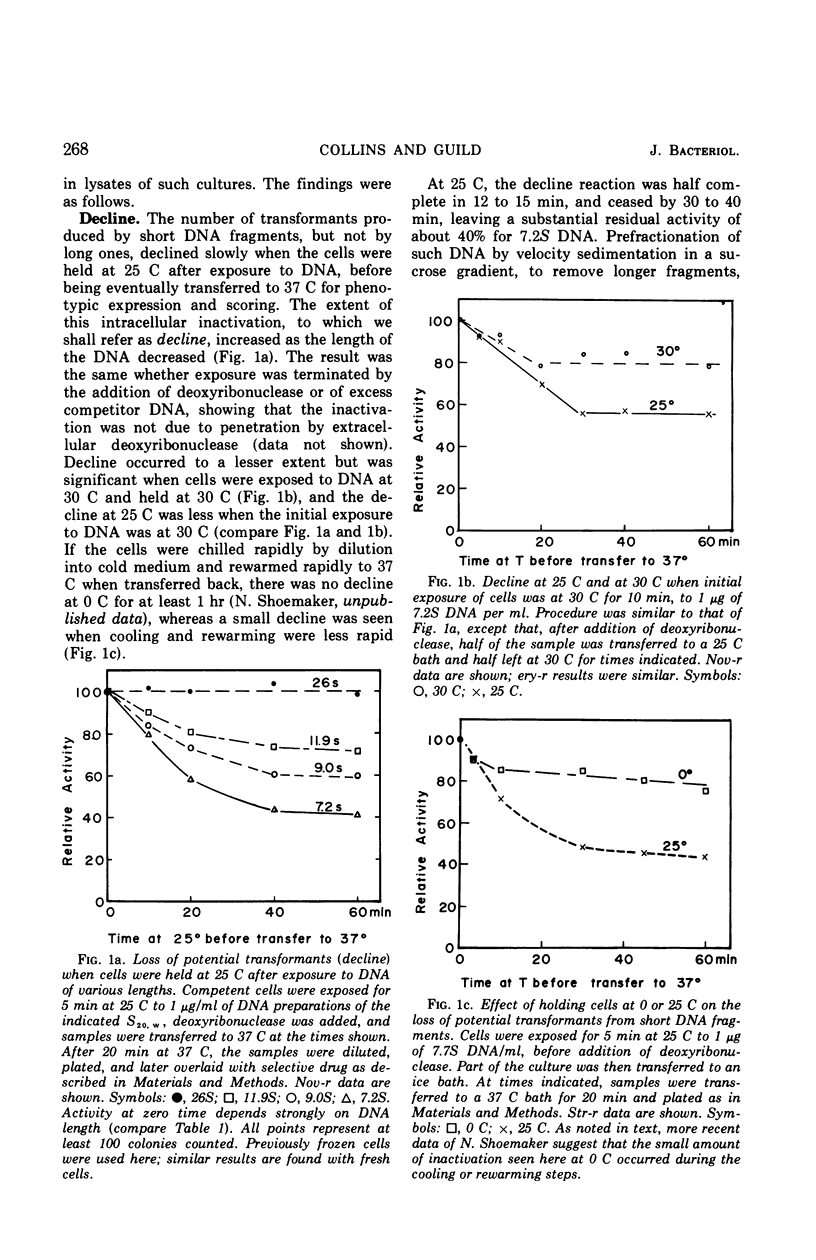
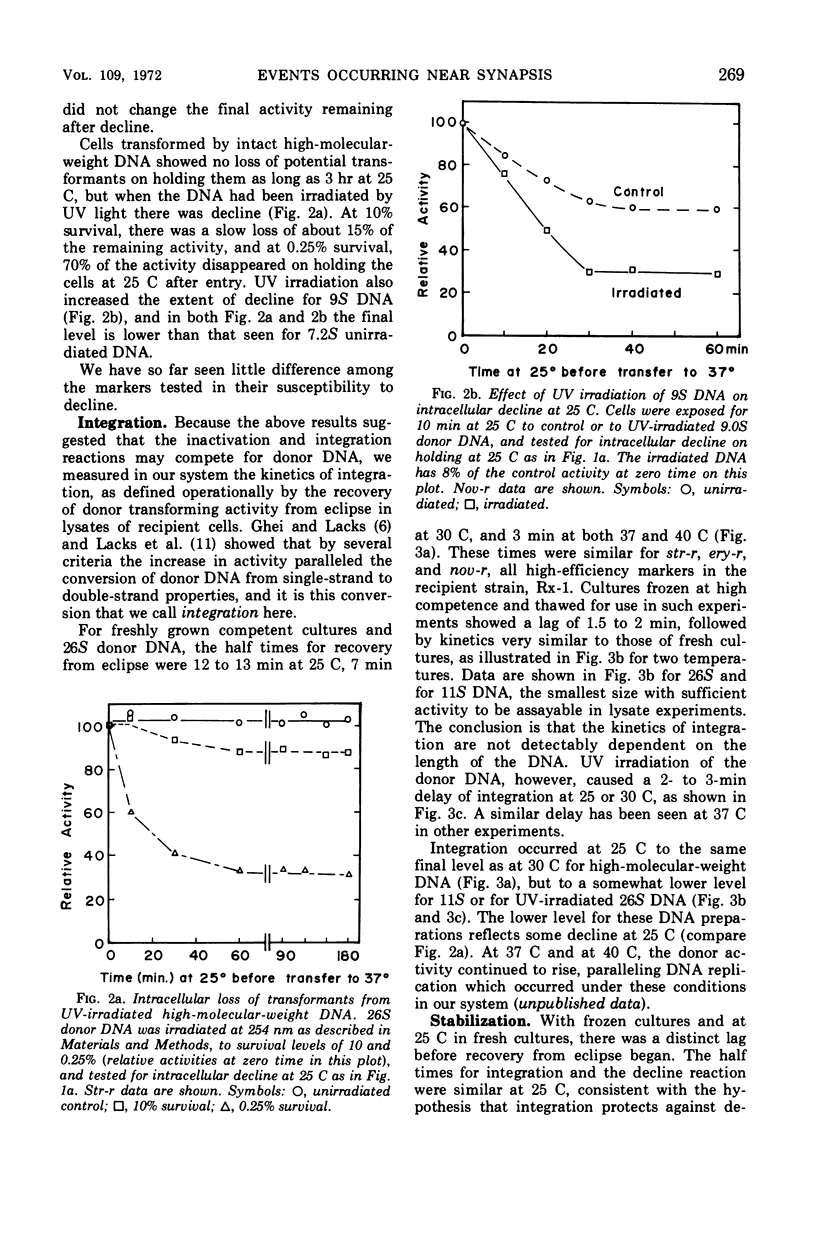
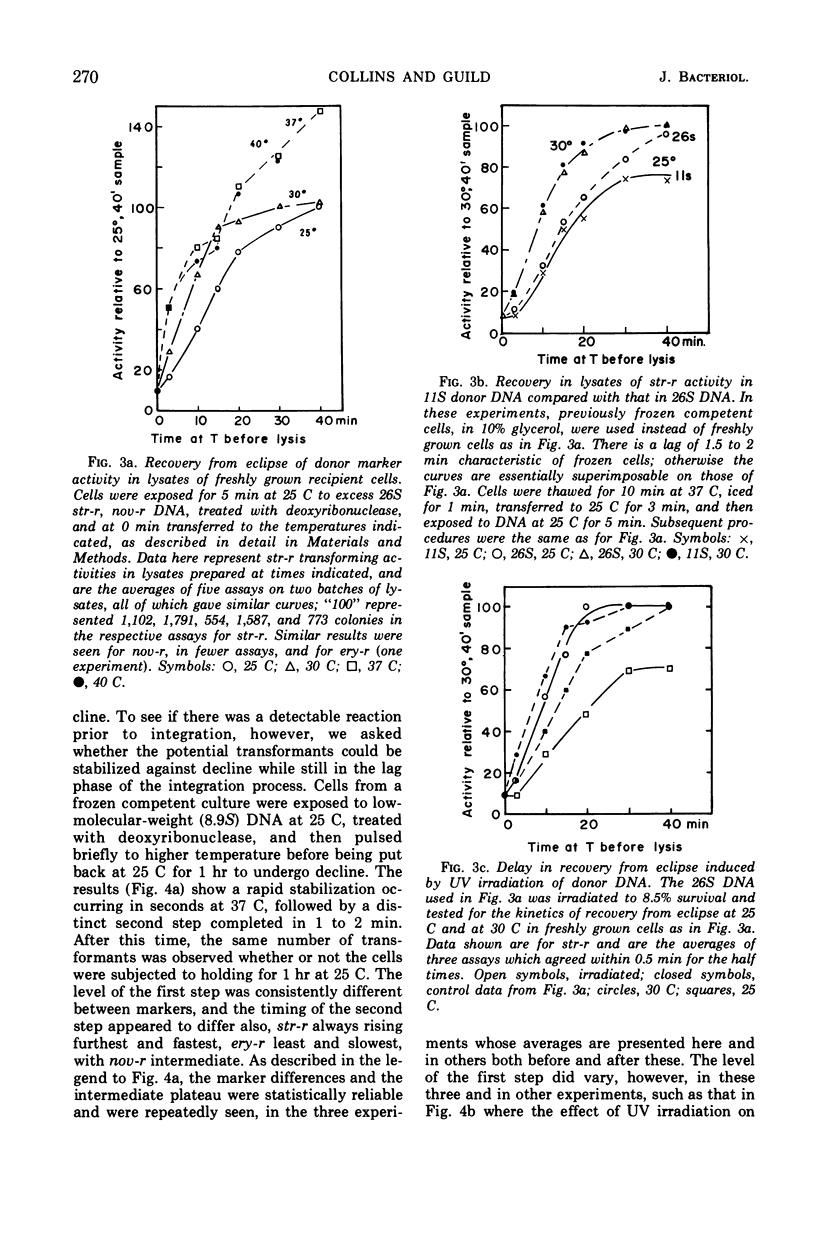
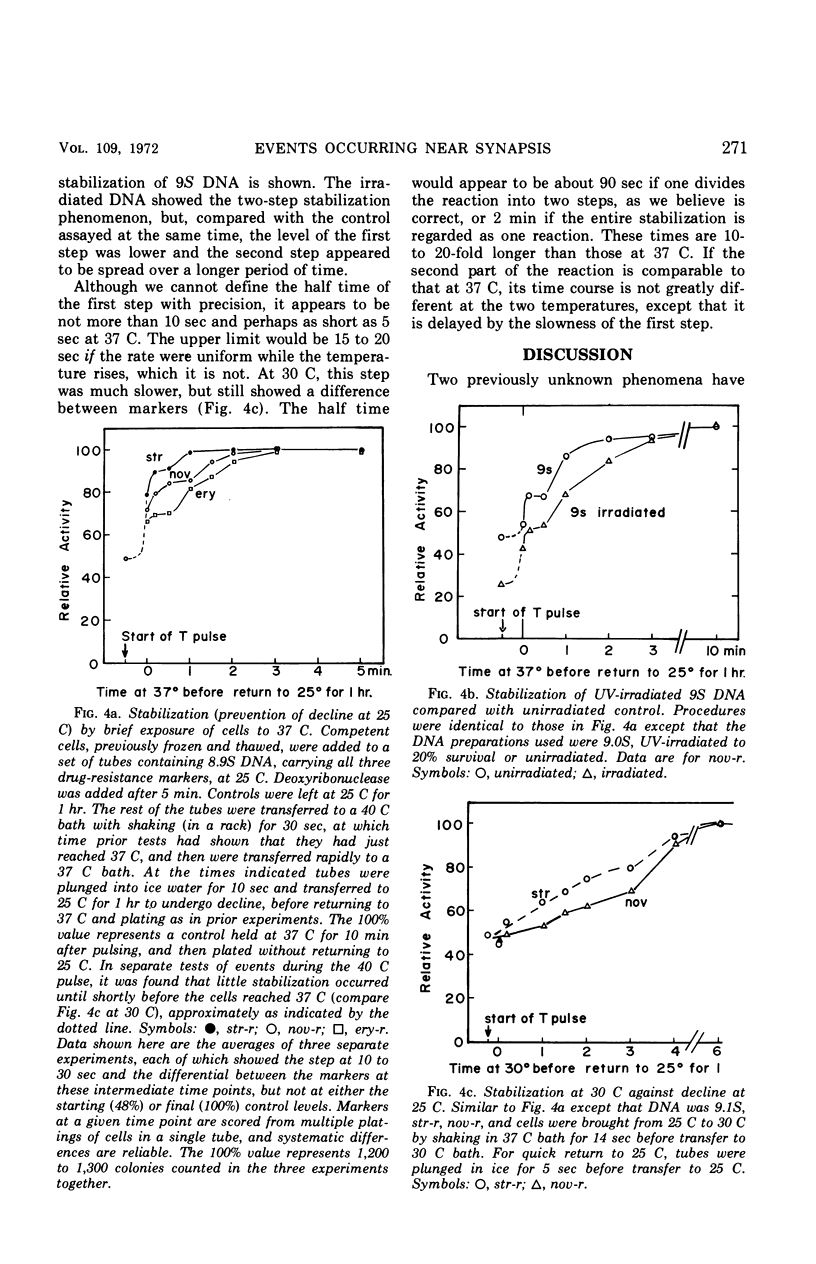
A marker-specific and strongly temperature-dependent reaction was observed to occur at a time during transformation in Diplococcus pneumoniae after the donor deoxyribonucleic acid (DNA) had acquired single-strand properties and immediately preceding the integration of these strands into the recipient chromosome. Operationally, it was observed as the prevention of an intracellular inactivation process, also described in this paper, which is specific for low molecular weight or for damaged DNA, and which occurs if the recipient cells are held at suboptimal temperatures after the DNA has entered. Brief exposure of the cells to a higher temperature stabilized the DNA against this inactivation, in a two step process. It is the first step which has a strong temperature dependence (ΔH‡ = 70 kcal/mole, ΔS‡ = 160 entropy units), is marker specific, and which appears to be reversible. The second step is much less temperature-dependent and overlaps in time the start of integration. The enthalpy and entropy of activation are both consistent with those needed to open a loop of six to eight base pairs in a DNA duplex. It is suggested that these observations may reflect, and provide an assay for, the kinetics of synapsis, which on this model is limited in rate by the appearance of unpaired regions on the recipient duplex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B. M., Frey L. T4 bacteriophage gene 32: a structural protein in the replication and recombination of DNA. Nature. 1970 Sep 26;227(5265):1313–1318. doi: 10.1038/2271313a0. [DOI] [PubMed] [Google Scholar]
- Carrier W. L., Setlow R. B. Endonuclease from Micrococcus luteus which has activity toward ultraviolet-irradiated deoxyribonucleic acid: purification and properties. J Bacteriol. 1970 Apr;102(1):178–186. doi: 10.1128/jb.102.1.178-186.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A., Jr, Guild W. R. Transformation and DNA size. I. Activity of fragments of defined size and a fit to a random double cross-over model. J Mol Biol. 1968 Oct 14;37(1):157–178. doi: 10.1016/0022-2836(68)90080-6. [DOI] [PubMed] [Google Scholar]
- FOX M. S., ALLEN M. K. ON THE MECHANISM OF DEOXYRIBONUCLEATE INTEGRATION IN PNEUMOCOCCAL TRANSFORMATION. Proc Natl Acad Sci U S A. 1964 Aug;52:412–419. doi: 10.1073/pnas.52.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILD W. R., ROBINSON M. Evidence for message reading from a unique strand of pneumococcal DNA. Proc Natl Acad Sci U S A. 1963 Jul;50:106–112. doi: 10.1073/pnas.50.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghei O. K., Lacks S. A. Recovery of donor deoxyribonucleic acid marker activity from eclipse in pneumococcal transformation. J Bacteriol. 1967 Mar;93(3):816–829. doi: 10.1128/jb.93.3.816-829.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M. Incorporation of oligodeoxynucleotides into DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):284–291. doi: 10.1073/pnas.61.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild W. R., Cato A., Jr, Lacks S. Transformation and DNA size: two controlling parameters and the efficiency of the single strand intermediate. Cold Spring Harb Symp Quant Biol. 1968;33:643–645. doi: 10.1101/sqb.1968.033.01.072. [DOI] [PubMed] [Google Scholar]
- LACKS S. Molecular fate of DNA in genetic transformation of Pneumococcus. J Mol Biol. 1962 Jul;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Guild W. R. Number of transformable units per cell in Diplococcus pneumoniae. J Bacteriol. 1969 Mar;97(3):1033–1035. doi: 10.1128/jb.97.3.1033-1035.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Scheffler I. E., Sturtevant J. M. Thermodynamics of the helix-coil transition of the alternating copolymer of deoxyadenylic acid and deoxythymidylic acid. J Mol Biol. 1969 Jun 28;42(3):577–580. doi: 10.1016/0022-2836(69)90244-7. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Randolph M. L., Boling M. E., Mattingly A., Price G., Gordon M. P. Repair of DNA in Haemophilus influenzae. II. Excision, repair of single-strand breaks, defects in transformation, and host cell modification in UV-sensitive mutants. Cold Spring Harb Symp Quant Biol. 1968;33:209–218. doi: 10.1101/sqb.1968.033.01.024. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Printz M. P. Dynamic aspects of DNA structure as studies by hydrogen exchange. Fed Proc. 1965 Nov-Dec;24(6):1458–1465. [PubMed] [Google Scholar]
- Wang J. C., Davidson N. On the probability of ring closure of lambda DNA. J Mol Biol. 1966 Aug;19(2):469–482. doi: 10.1016/s0022-2836(66)80017-7. [DOI] [PubMed] [Google Scholar]



