Abstract
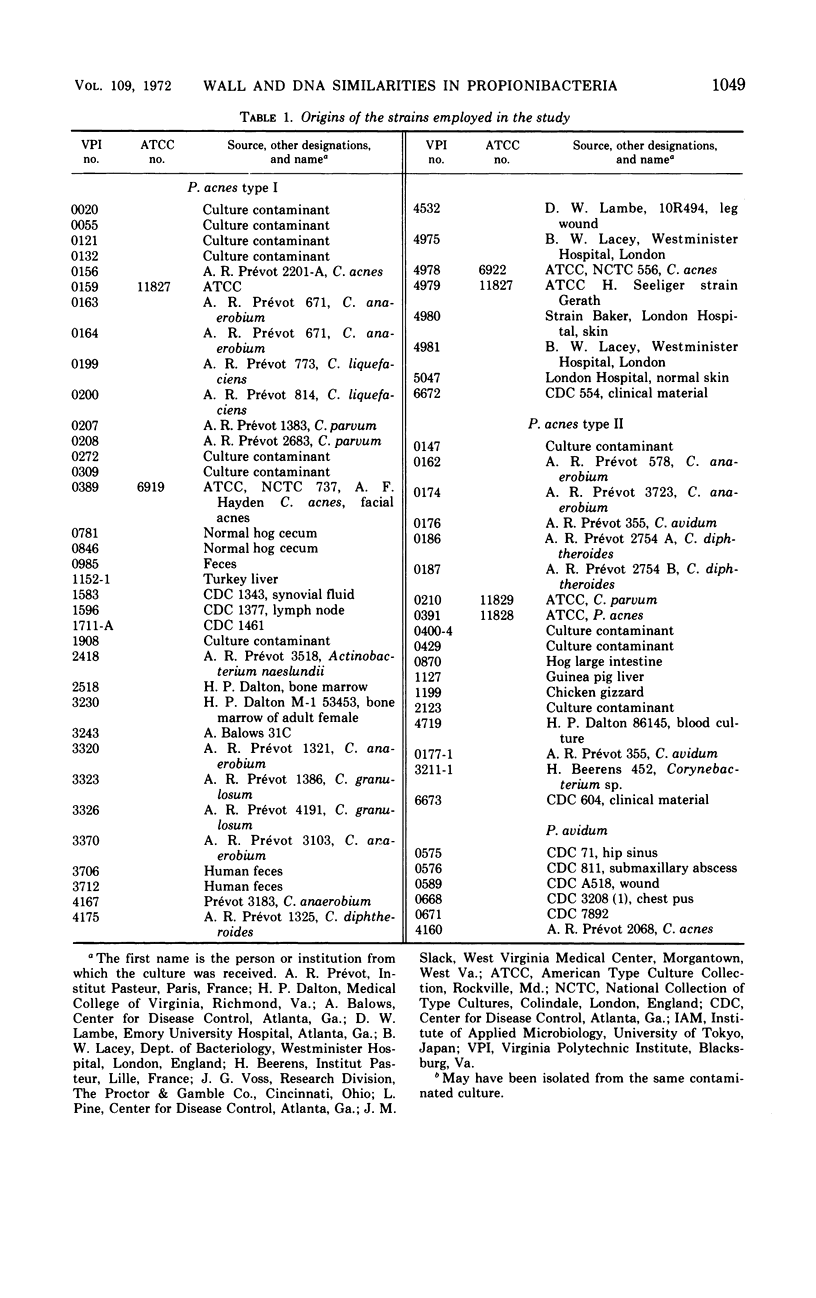
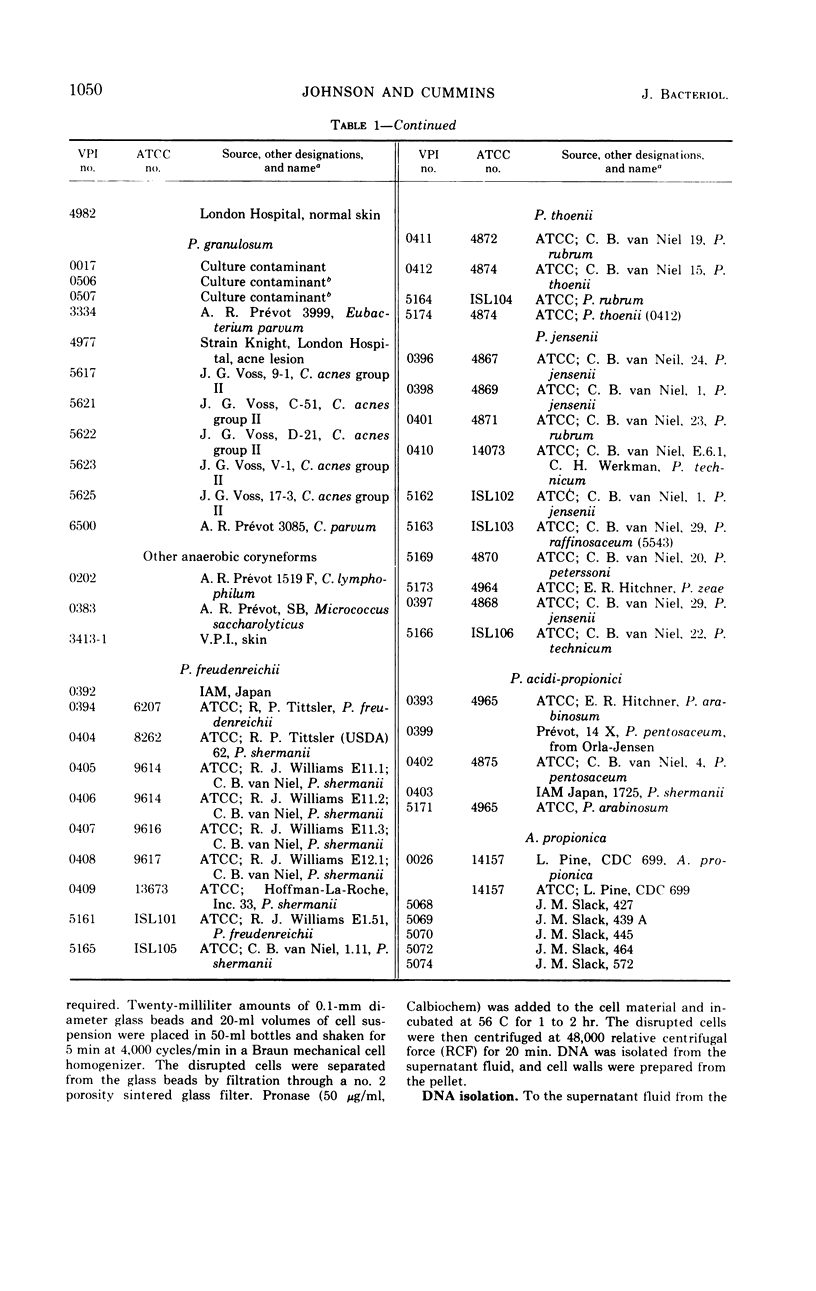
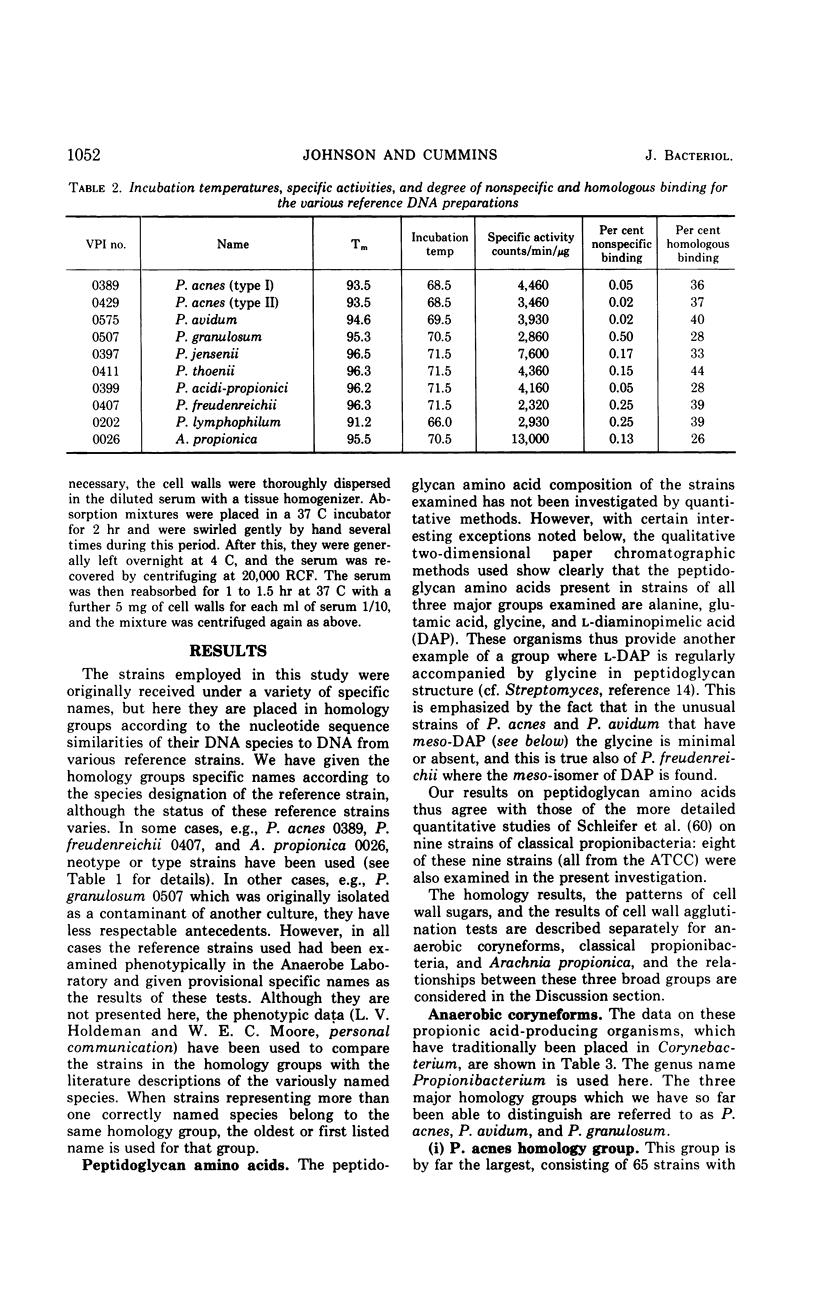
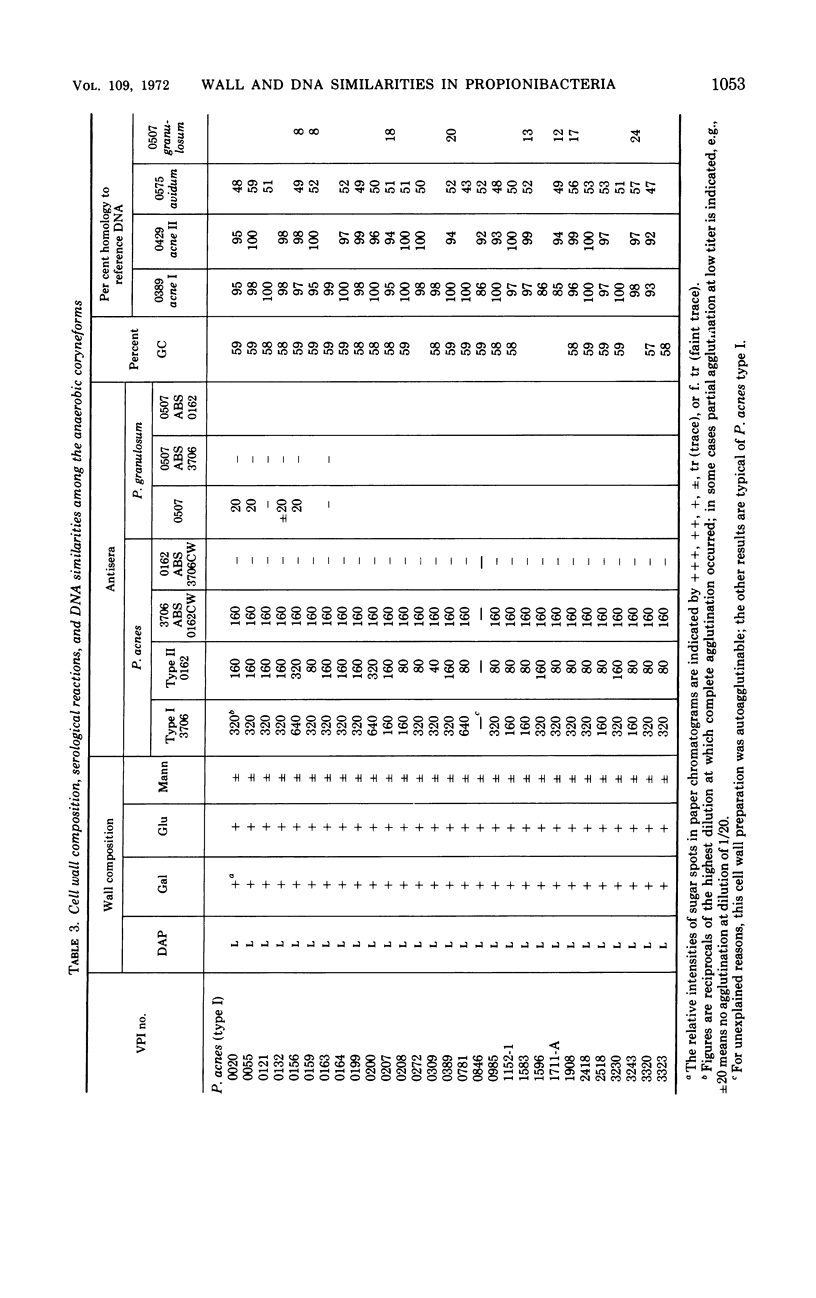
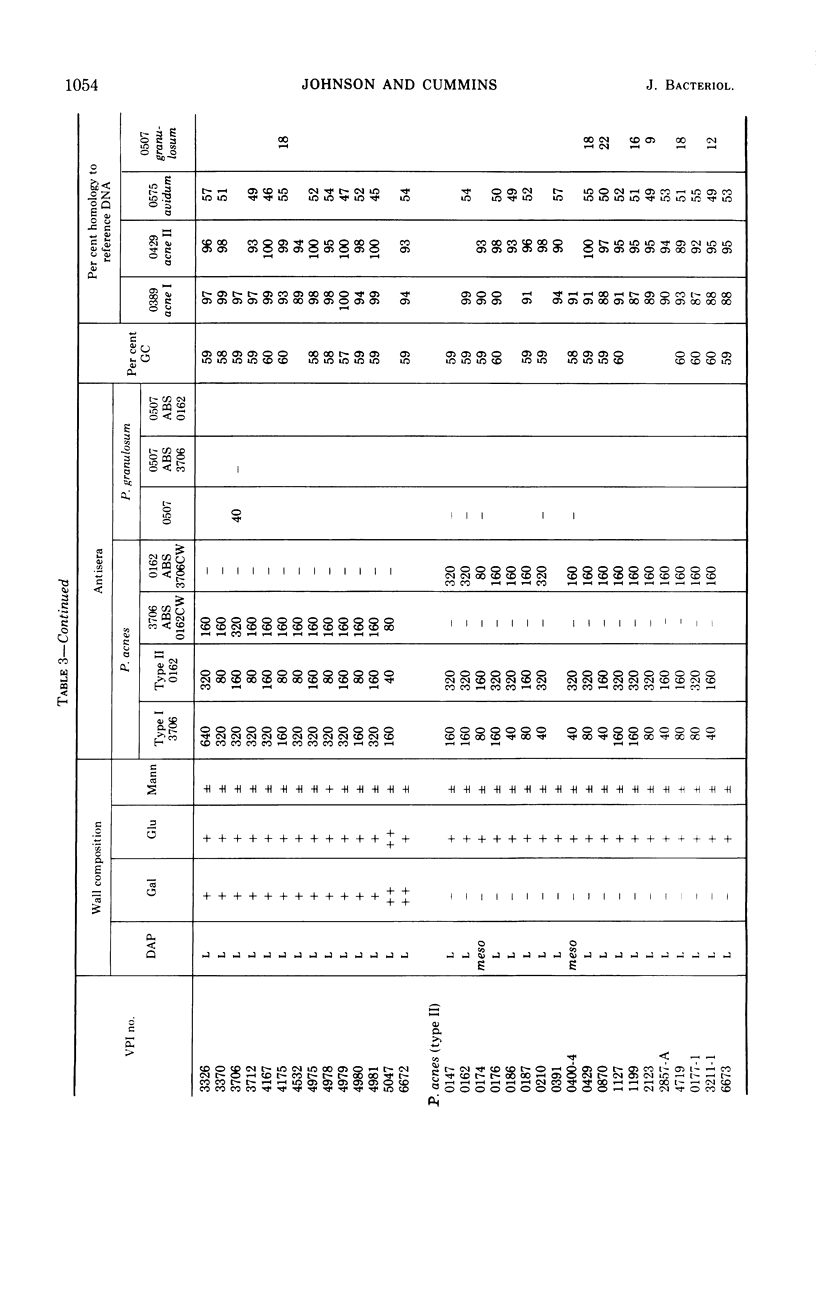
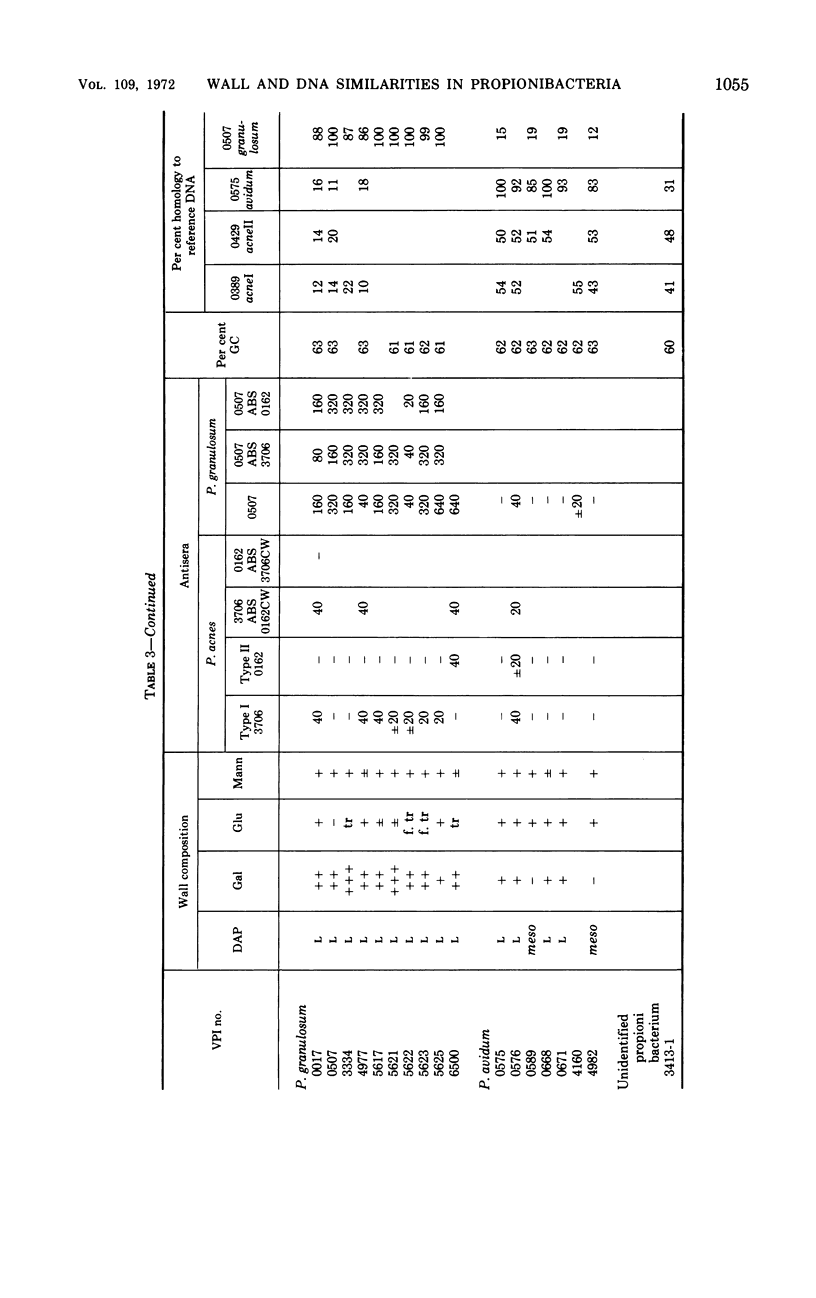
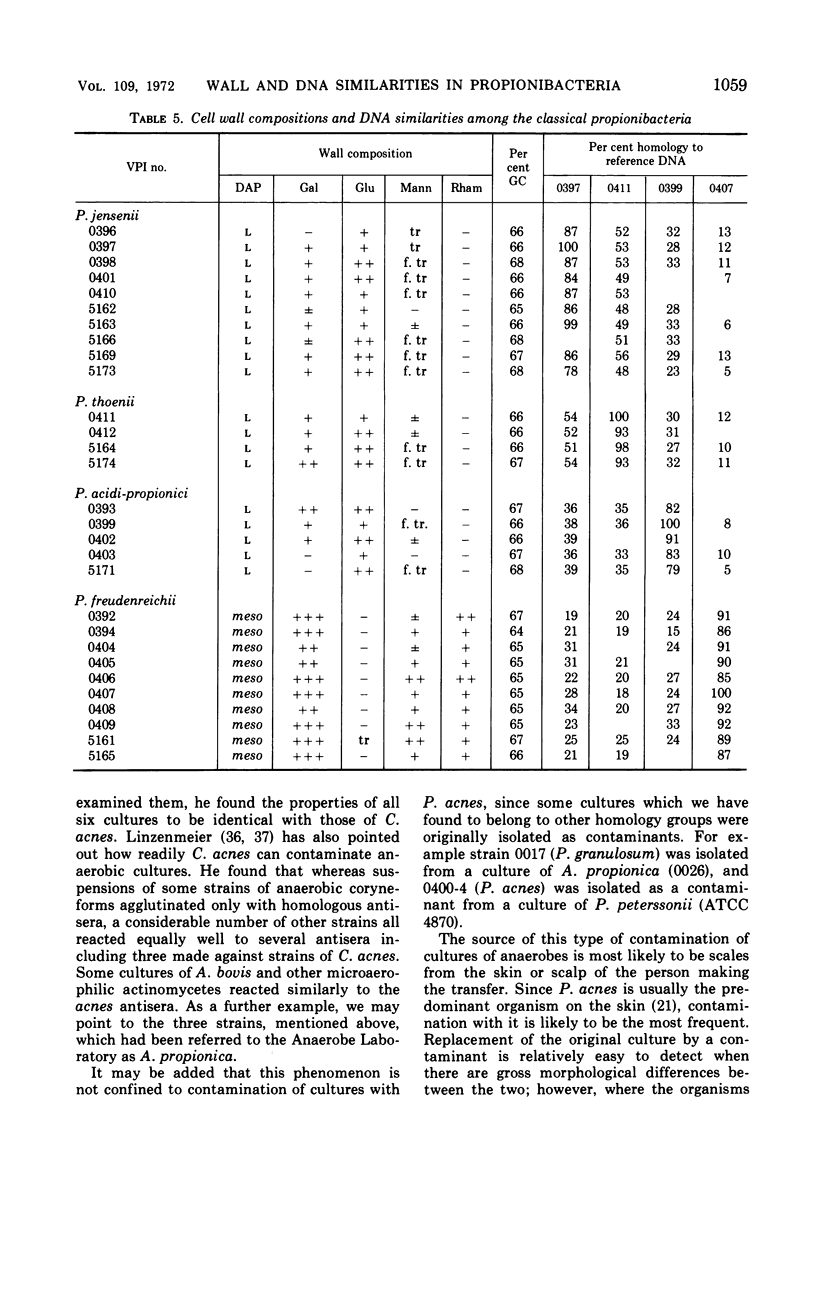
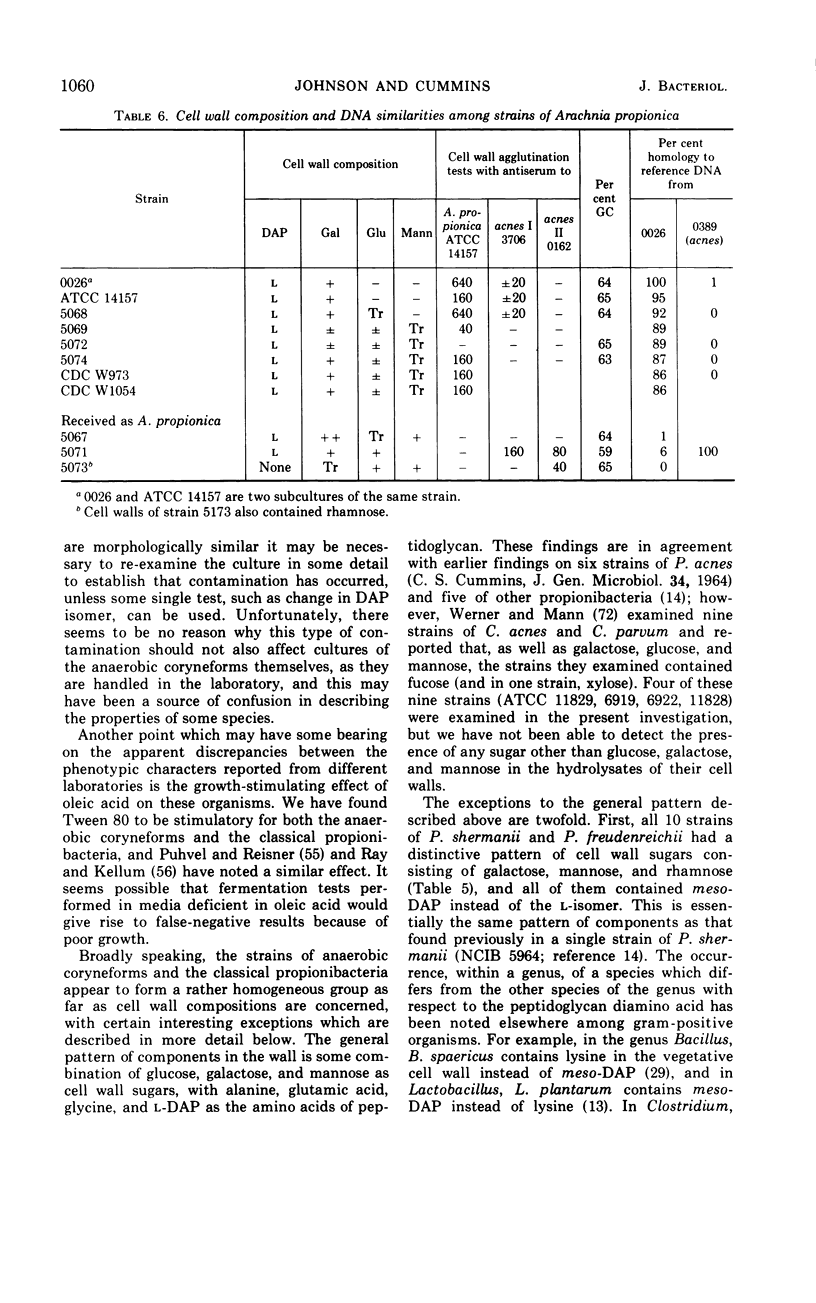
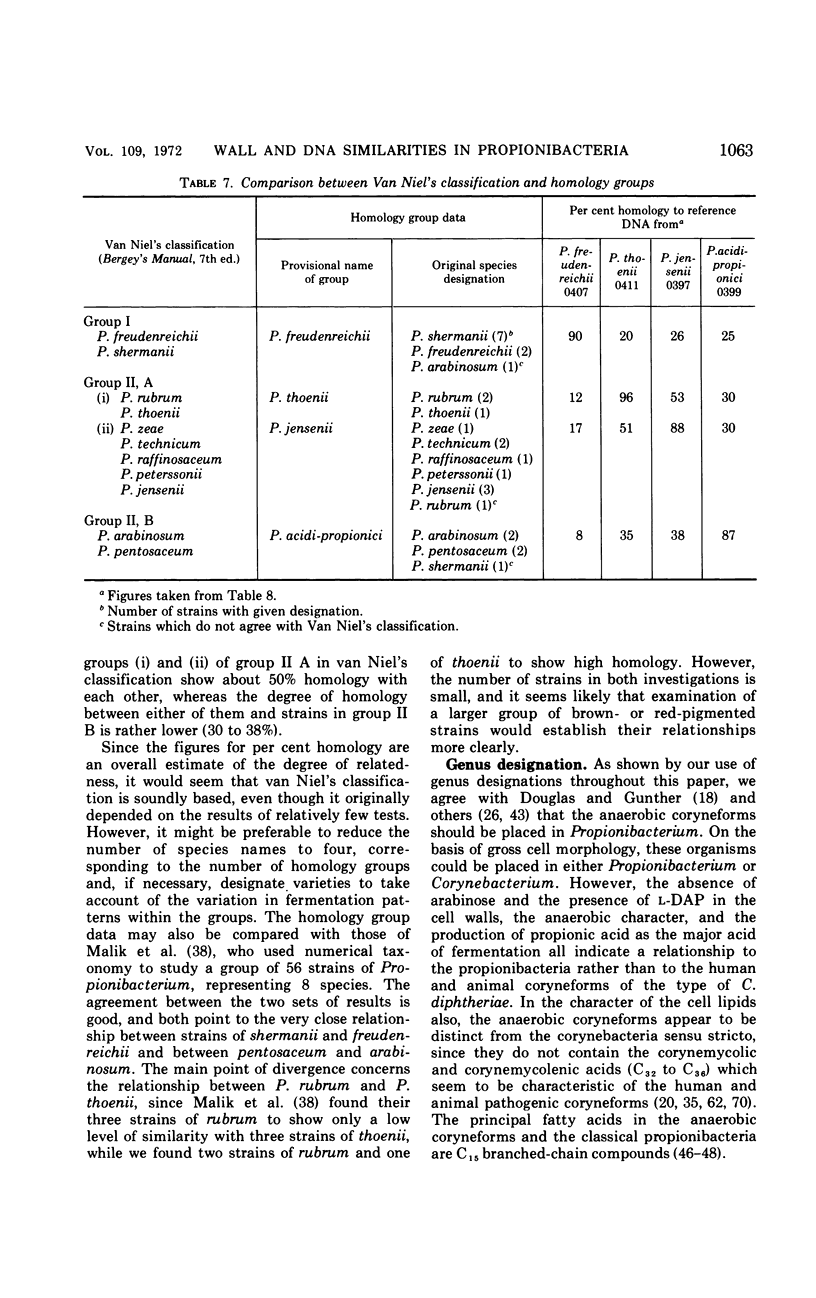
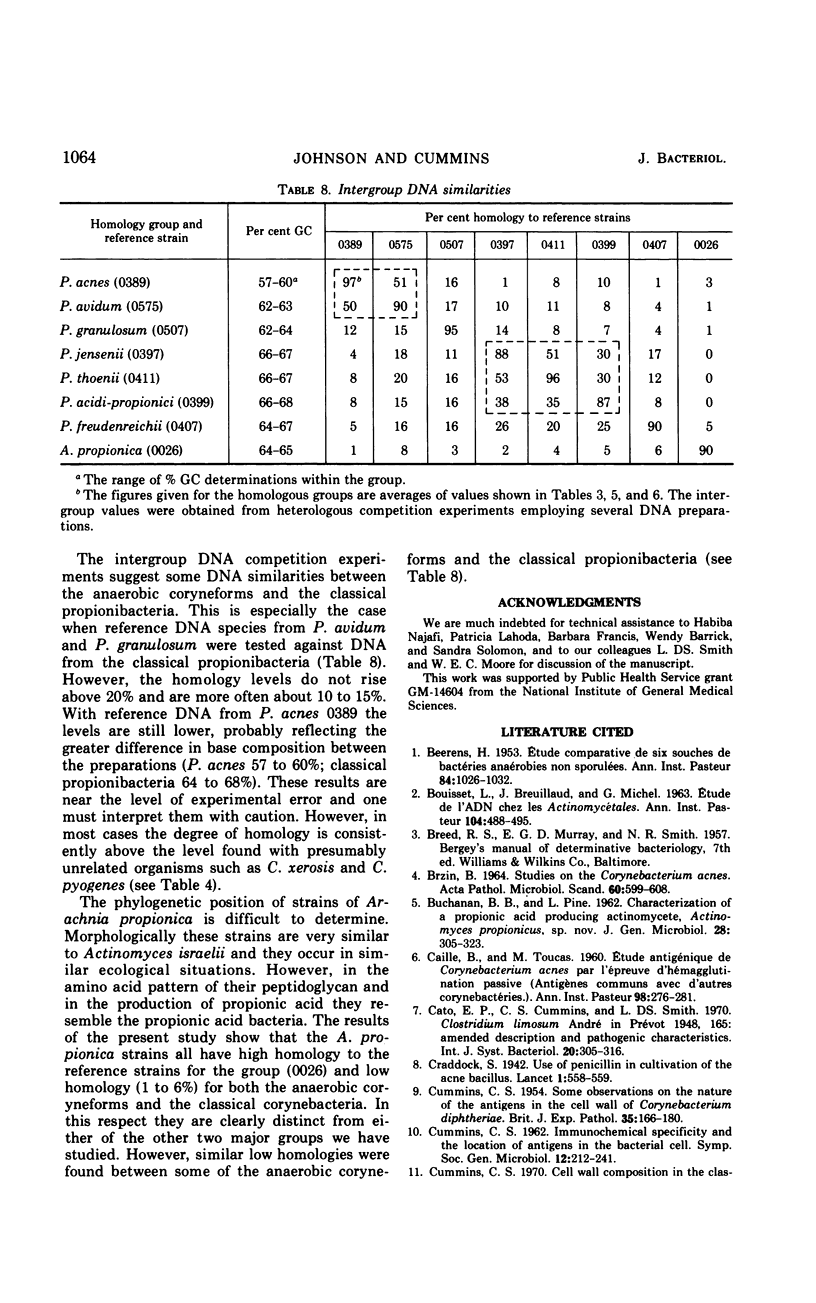
Eighty strains of anaerobic coryneforms were compared with 29 strains of classical propionibacteria and 8 strains of Arachnia propionica by cell wall analysis, deoxyribonucleic acid (DNA) base compositions, and nucleotide sequence similarities. The anaerobic coryneforms have DNA base compositions in the range of 58 to 64% guanine + cytosine (GC) and show at least three homology groups. The largest group corresponds to organisms identified as Propionibacterium acnes and shows about 50% homology to strains in the P. avidum homology group. The third group, P. granulosum, shows low levels of similarities to the other two. All strains of anaerobic coryneforms have some combination of galactose, glucose, or mannose as cell wall sugars, and most have alanine (ala), glutamic acid (glu), glycine (gly), and l-α-ε-diaminopimelic acid (l-DAP) as amino acids of peptidoglycan. However, a few strains in the P. acnes and P. avidum homology groups have meso-DAP and minimal amounts of glycine. Two serological types, based on cell wall antigens, were found in the P. acnes homology group. One type had galactose, glucose, and mannose as cell wall sugars, the other glucose and mannose only. The classical propionibacteria have DNA base compositions in the range of 65 to 68% GC and show four homology groups which correspond closely to van Niel's classification as given in the 7th edition of Bergey's Manual. The P. jensenii group showed about 50% homology to the P. thoenii group and about 30 to 35% to the P. acidi-propionici group. The P. freudenreichii strains showed a rather lower level of similarity (8 to 25%) to the other homology groups. Most of the strains of classical propionibacteria also have some combination of galactose, glucose, or mannose as cell wall sugars and ala, glu, gly, and l-DAP as peptidoglycan amino acids, but P. shermanii and P. freudenreichii strains, which form a single homology group, have galactose, mannose, and rhamnose as cell wall sugars and ala, glu, and meso-DAP in their peptidoglycan. There is a rather low level of DNA homology (10 to 20%) between the anaerobic coryneforms and classical propionibacteria. However, the strains of A. propionica which have a GC content of 64 to 65% and form a single homology group, show no homology to either of the other two major groups.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERENS H. Etude comparative de six souches de bactéries anaérobies non sporulées: Actinomyces bovis A.T.C.C 8373 (bovine), A. bovis A.T.C.C. 8374 (humaine), A. israeli var. liquefaciens, Corynebacterium acnes, Corynebacterium avidum, Corynebacterium liquefaciens. Ann Inst Pasteur (Paris) 1953 Jun;84(6):1026–1032. [PubMed] [Google Scholar]
- BOUISSET L., BREUILLAUD J., MICHEL G. [Study of DNA in Actinomycetes. Corynebacterium DNA]. Ann Inst Pasteur (Paris) 1963 Apr;104:488–495. [PubMed] [Google Scholar]
- BRZIN B. STUDIES ON THE CORYNEBACTERIUM ACNES. Acta Pathol Microbiol Scand. 1964;60:599–608. doi: 10.1111/apm.1964.60.4.599. [DOI] [PubMed] [Google Scholar]
- BUCHANAN B. B., PINE L. Characterization of a propionic acid producing actinomycete, Actinomyces propionicus, sp. nov. J Gen Microbiol. 1962 Jun;28:305–323. doi: 10.1099/00221287-28-2-305. [DOI] [PubMed] [Google Scholar]
- CAILLE B., TOUCAS M. [Antigenic study of Corynebacterium acnes by the passive hemagglutination test. Antigens in common with other Corynebacteria]. Ann Inst Pasteur (Paris) 1960 Feb;98:276–281. [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. Studies on the cell-wall composition and taxonomy of Actinomycetales and related groups. J Gen Microbiol. 1958 Feb;18(1):173–189. doi: 10.1099/00221287-18-1-173. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S. Some observations on the nature of the antigens in the cell wall of Corynebacterium diphtheriae. Br J Exp Pathol. 1954 Apr;35(2):166–180. [PMC free article] [PubMed] [Google Scholar]
- Cummins C. S. Catalase activity in Corynebacterium pyogenes. Can J Microbiol. 1971 Jul;17(7):1001–1002. doi: 10.1139/m71-158. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Doty P., Marmur J., Eigner J., Schildkraut C. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: PHYSICAL CHEMICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):461–476. doi: 10.1073/pnas.46.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas H. C., Gunter S. E. The Taxonomic Position of Corynebacterium acnes. J Bacteriol. 1946 Jul;52(1):15–23. [PMC free article] [PubMed] [Google Scholar]
- ETEMADI A. H. ISOLEMENT DES ACIDES ISOPENTAD'ECANOUIQUE ET ISOHEPTAD'ECANOUIQUE DES LIPIDES DE CORYNEBACTERIUM PARVUM. Bull Soc Chim Biol (Paris) 1963;45:1423–1432. [PubMed] [Google Scholar]
- EVANS C. A., SMITH W. M., JOHNSTON E. A., GIBLETT E. R. Bacterial flora of the normal human skin. J Invest Dermatol. 1950 Oct;15(4):305–324. doi: 10.1038/jid.1950.105. [DOI] [PubMed] [Google Scholar]
- Eggerth A. H. The Gram-positive Non-spore-bearing Anaerobic Bacilli of Human Feces. J Bacteriol. 1935 Sep;30(3):277–299. doi: 10.1128/jb.30.3.277-299.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTIERREZ J. Numbers and characteristics of lactate-utilizing organisms in the rumen of cattle. J Bacteriol. 1953 Aug;66(2):123–128. doi: 10.1128/jb.66.2.123-128.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerencser M. A., Slack J. M. Isolation and characterization of Actinomyces propionicus. J Bacteriol. 1967 Jul;94(1):109–115. doi: 10.1128/jb.94.1.109-115.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Hitchner E. R. Some Physiological Characteristics of the Propionic-Acid Bacteria. J Bacteriol. 1934 Nov;28(5):473–479. doi: 10.1128/jb.28.5.473-479.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Anderson R. S., Ordal E. J. Nucleic acid homologies among oxidase-negative Moraxella species. J Bacteriol. 1970 Feb;101(2):568–573. doi: 10.1128/jb.101.2.568-573.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. L., Ordal E. J. Deoxyribonucleic acid homology in bacterial taxonomy: effect of incubation temperature on reaction specificity. J Bacteriol. 1968 Mar;95(3):893–900. doi: 10.1128/jb.95.3.893-900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINZENMEIER G. Etude sérologique des corynébactéries anaérobies par la methode des agglutinines. Ann Inst Pasteur (Paris) 1954 Nov;87(5):572–579. [PubMed] [Google Scholar]
- LINZENMEIER G. Serologie anaerober Corynebakterien. II. Serologie und pathogenetische Bedeutung von Corynebacterium acnes. Zentralbl Bakteriol Orig. 1957 Nov;170(1-5):85–90. [PubMed] [Google Scholar]
- Lacave C., Asselineau J., Toubiana R. Sur quelques constituants lipidiques de Corynebacterium ovis. Eur J Biochem. 1967 Jul;2(1):37–43. doi: 10.1111/j.1432-1033.1967.tb00102.x. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. An approach to the measurement of genetic relatedness among organisms. Proc Natl Acad Sci U S A. 1963 Jul;50:156–164. doi: 10.1073/pnas.50.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE W. E., CATO E. P. VALIDITY OF PROPIONIBACTERIUM ACNES (GILCHRIST) DOUGLAS AND GUNTER COMB. NOV. J Bacteriol. 1963 Apr;85:870–874. doi: 10.1128/jb.85.4.870-874.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik A. C., Reinbold G. W., Vedamuthu E. R. An evaluation of the taxonomy of Propionibacterium. Can J Microbiol. 1968 Nov;14(11):1185–1191. doi: 10.1139/m68-199. [DOI] [PubMed] [Google Scholar]
- Moss C. W., Cherry W. B. Characterization of the C15 branched-chain fatty acids of Corynebacterium acnes by gas chromatography. J Bacteriol. 1968 Jan;95(1):241–242. doi: 10.1128/jb.95.1.241-242.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dowell V. R., Jr, Farshtchi D., Raines L. J., Cherry W. B. Cultural characteristics and fatty acid composition of propionibacteria. J Bacteriol. 1969 Feb;97(2):561–570. doi: 10.1128/jb.97.2.561-570.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Dowell V. R., Jr, Lewis V. J., Schekter M. A. Cultural characteristics and fatty acid composition of Corynebacterium acnes. J Bacteriol. 1967 Nov;94(5):1300–1305. doi: 10.1128/jb.94.5.1300-1305.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE L., HARDIN H. Actinomyces israelii, a cause of lacrimal canaliculitis in man. J Bacteriol. 1959 Aug;78:164–170. doi: 10.1128/jb.78.2.164-170.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhvel S. M., Reisner R. M. Effect of fatty acids on the growth of Corynebacterium acnes in vitro. J Invest Dermatol. 1970 Jan;54(1):48–52. doi: 10.1111/1523-1747.ep12551667. [DOI] [PubMed] [Google Scholar]
- Ray L. F., Kellum R. E. Corynebacterium acnes from human skin. Identification by morphologic, cultural, biochemical, serological, and chromatographic methods. Arch Dermatol. 1970 Jan;101(1):36–40. doi: 10.1001/archderm.101.1.36. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SEBALD M., GASSER F., WERNER H. TENEUR GC PERCENTAGE ET CLASSIFICATION. APPLICATION AU GROUPE DES BIFIDOBACT'ERIES ET 'A QUELQUES GENRES VOISINS. Ann Inst Pasteur (Paris) 1965 Aug;109:251–269. [PubMed] [Google Scholar]
- Schleifer K. H., Plapp R., Kandler O. Glycine as crosslinking bridge in the LL-diaminopimelic acid containing murein of Propionibacterium peterssonii. FEBS Lett. 1968 Oct;1(5):287–290. doi: 10.1016/0014-5793(68)80133-4. [DOI] [PubMed] [Google Scholar]
- Senn M., Ioneda T., Pudles J., Lederer E. Spectrométrie de masse de glycolipides. I. Structure du "cord factor" de Corynebacterium diphtheriae. Eur J Biochem. 1967 May;1(3):353–356. doi: 10.1111/j.1432-1033.1967.tb00081.x. [DOI] [PubMed] [Google Scholar]
- Sherman J. M. The Cause of Eyes and Characteristic Flavor in Emmental or Swiss Cheese. J Bacteriol. 1921 Jul;6(4):379–393. doi: 10.1128/jb.6.4.379-393.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. F. Characterization of human cutaneous lipophilic diphtheroids. J Gen Microbiol. 1969 Mar;55(3):433–443. doi: 10.1099/00221287-55-3-433. [DOI] [PubMed] [Google Scholar]
- Voss J. G. Differentiation of two groups of Corynebacterium acnes. J Bacteriol. 1970 Feb;101(2):392–397. doi: 10.1128/jb.101.2.392-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welby-Gieusse M., Lanéelle M. A., Asselineau J. Structure des acides corynomycoliqes de Corynebacterium hofmanii et leur implication biogénétique. Eur J Biochem. 1970 Mar 1;13(1):164–167. doi: 10.1111/j.1432-1033.1970.tb00913.x. [DOI] [PubMed] [Google Scholar]
- Werner H., Mann S. Chemische Analyse der Zellwand von Corynebacterium acnes und C. parvum. Zentralbl Bakteriol Orig. 1968 May;206(4):486–499. [PubMed] [Google Scholar]



