Abstract
Nitrosomonas europaea, Nitrobacter agilis, Thiobacillus denitrificans, T. neapolitanus, and T. thioparus (all obligate autotrophic bacteria) have been grown in dialysis culture, on glucose salts media, in the absence of their specific inorganic energy source. Metabolic products for N. agilis grown on nitrite salts medium were identified as keto acids. Pyruvic acid inhibited this organism at 5 × 10−5m. Keto acids were not inhibitory for the thiobacilli grown on thiosulfate medium. However, when T. denitrificans was grown on glucose with dialysis, addition of 5 × 10−4m pyruvate inhibited growth. Thus, it appears pyruvate may be inhibitory for other autotrophs, as has been reported for T. thiooxidans.
Full text
PDF
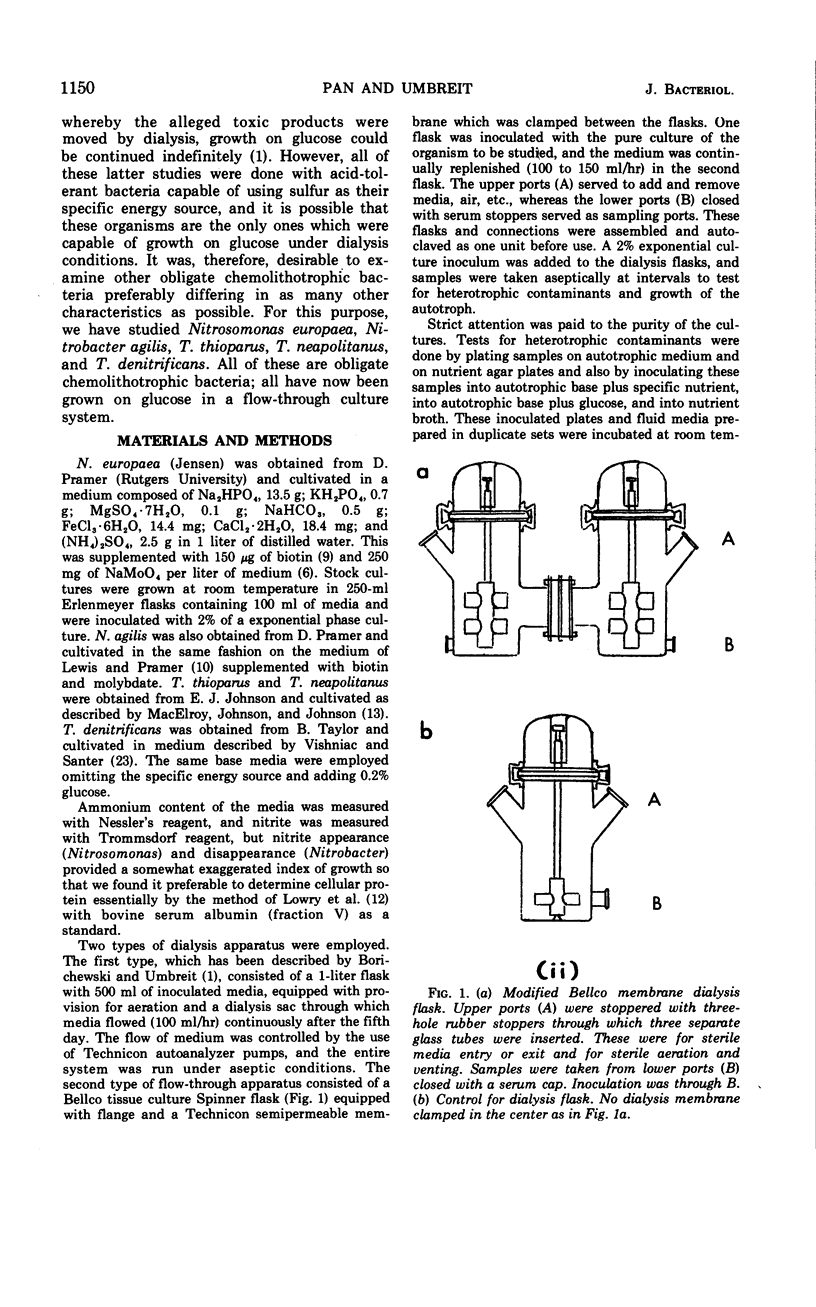
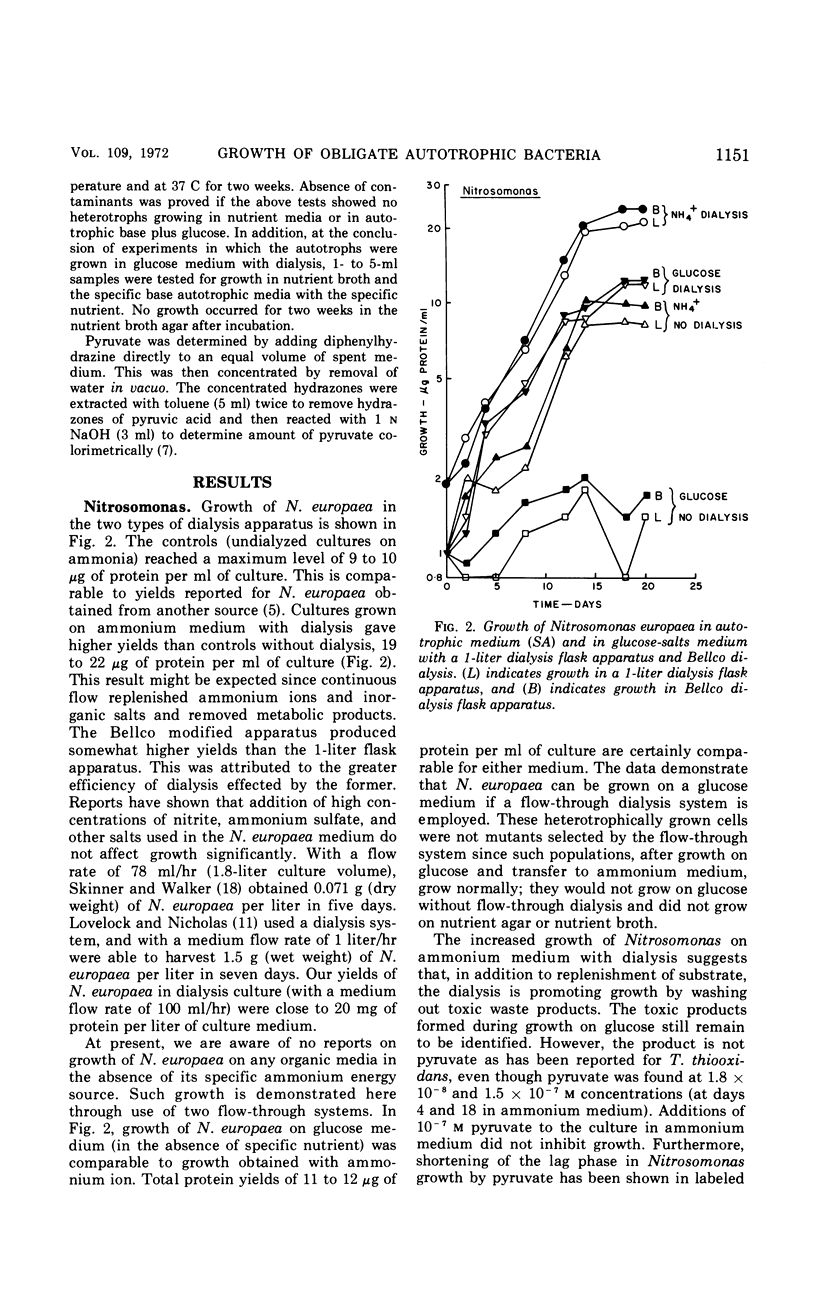
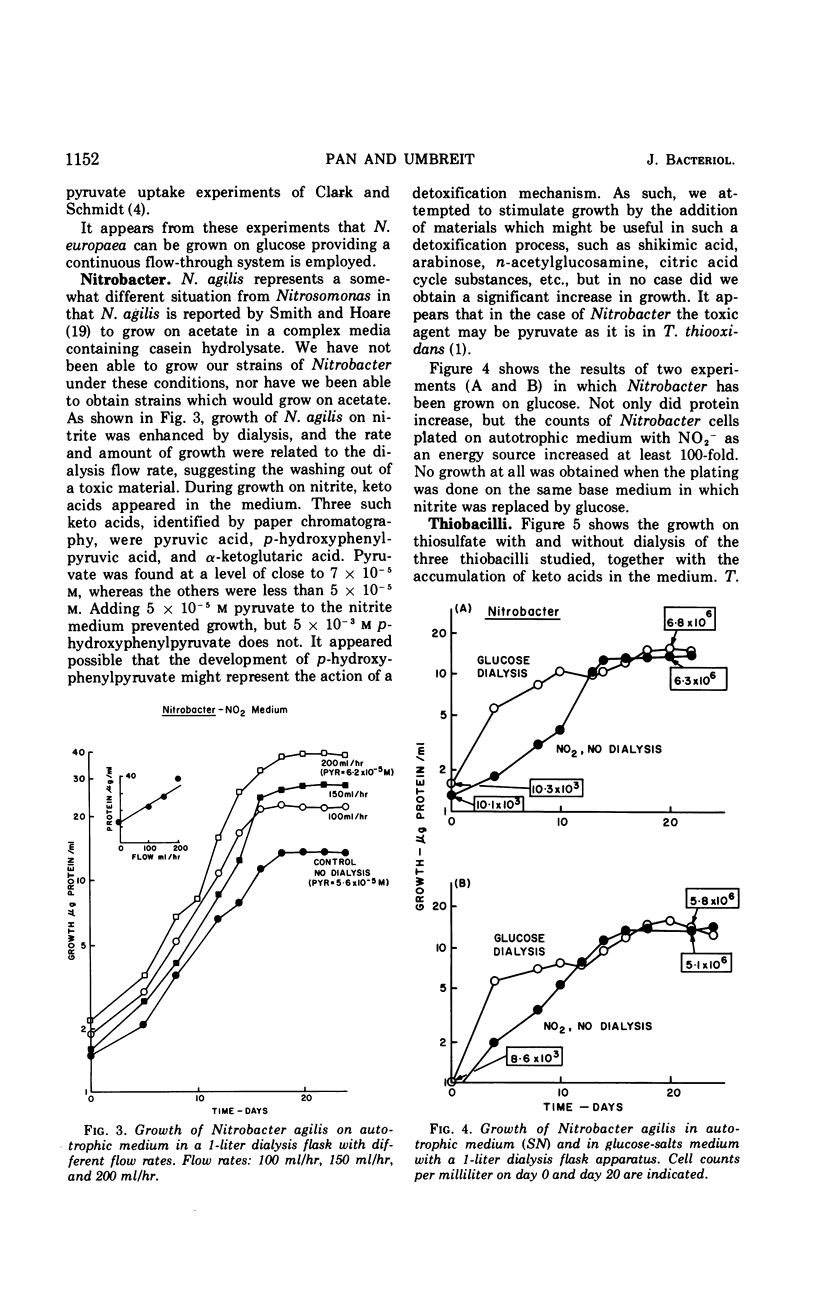
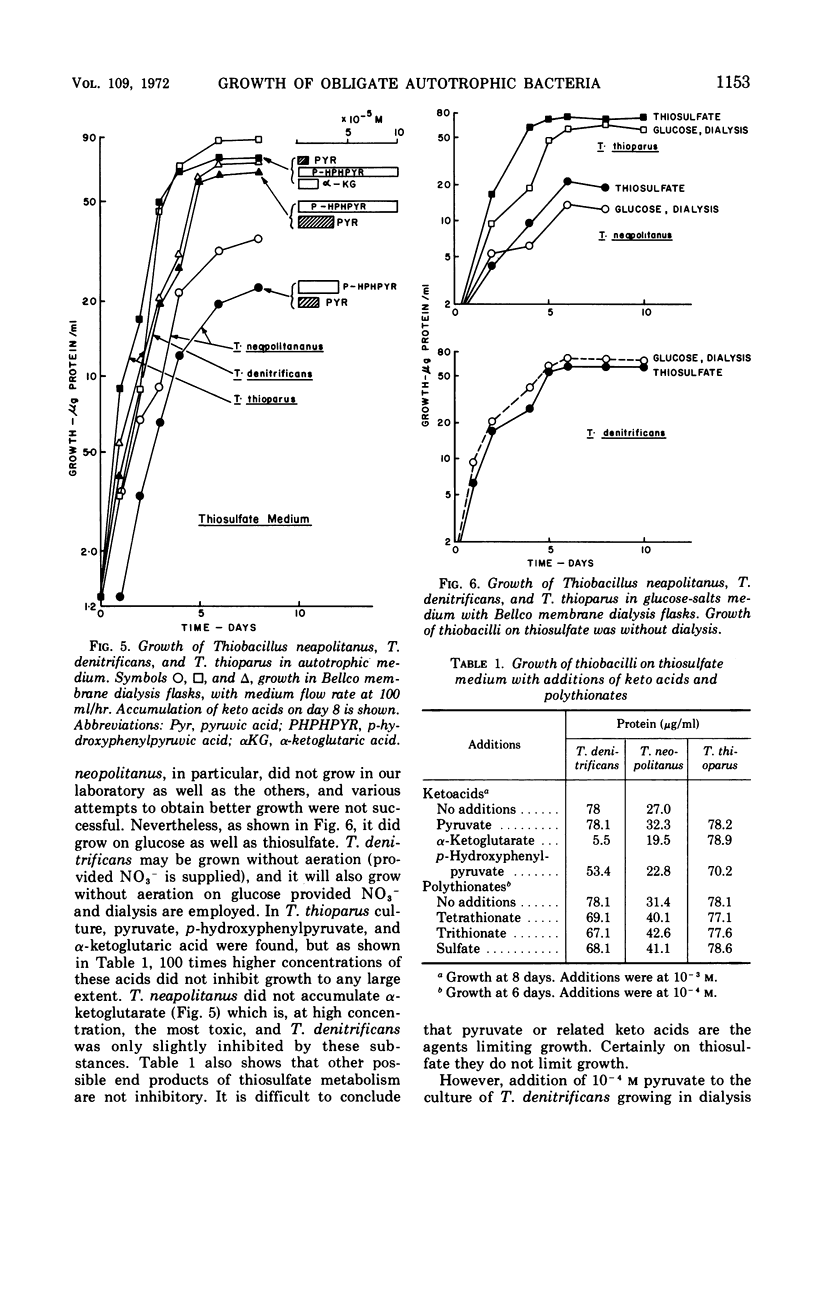

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borichewski R. M., Umbreit W. W. Growth of Thiobacillus thiooxidans on glucose. Arch Biochem Biophys. 1966 Sep 26;116(1):97–102. doi: 10.1016/0003-9861(66)90017-8. [DOI] [PubMed] [Google Scholar]
- Butler R. G., Umbreit W. W. Absorption and utilization of organic matter by the strict autotroph, Thiobacillus thiooxidans, with special reference to aspartic acid. J Bacteriol. 1966 Feb;91(2):661–666. doi: 10.1128/jb.91.2.661-666.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler R. G., Umbreit W. W. Reduced nicotinamide adenine dinucleotide oxidase and alpha-ketoglutaric dehydrogenase activity by Thiobacillus thiooxidans. J Bacteriol. 1969 Feb;97(2):966–967. doi: 10.1128/jb.97.2.966-967.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Effect of mixed culture on Nitrosomonas europaea simulated by uptake and utilization of pyruvate. J Bacteriol. 1966 Jan;91(1):367–373. doi: 10.1128/jb.91.1.367-373.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINSTEIN M. S., DELWICHE C. C. MOLYBDENUM AS A MICRONUTRIENT FOR NITROBACTER. J Bacteriol. 1965 Jan;89:123–128. doi: 10.1128/jb.89.1.123-128.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempfling W. P., Vishniac W. Oxidative phosphorylation in extracts of thiobacillus X. Biochem Z. 1965 Aug 6;342(3):272–287. [PubMed] [Google Scholar]
- Krulwich T. A., Funk H. B. Stimulation of Nitrobacter agilis by Biotin. J Bacteriol. 1965 Sep;90(3):729–733. doi: 10.1128/jb.90.3.729-733.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS R. F., PRAMER D. Isolation of Nitrosomonas in pure culture. J Bacteriol. 1958 Nov;76(5):524–528. doi: 10.1128/jb.76.5.524-528.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovelock H. R., Nicholas D. J. Growth of Nitrosomonas europaea in batch and in continuous culture. Arch Mikrobiol. 1968 May 8;61(3):302–309. doi: 10.1007/BF00446615. [DOI] [PubMed] [Google Scholar]
- MacElroy R. D., Johnson E. J., Johnson M. K. Characterization of ribulose diphosphate carboxylase and phosphoribulokinase from Thiobacillus thioparus and Thiobacillus neapolitanus. Arch Biochem Biophys. 1968 Sep 20;127(1):310–316. doi: 10.1016/0003-9861(68)90231-2. [DOI] [PubMed] [Google Scholar]
- Matin A., Rittenberg S. C. Enzymes of carbohydrate metabolism in Thiobacillus species. J Bacteriol. 1971 Jul;107(1):179–186. doi: 10.1128/jb.107.1.179-186.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao G. S., Berger L. R. Basis of pyruvate inhibition in Thiobacillus thiooxidans. J Bacteriol. 1970 May;102(2):462–466. doi: 10.1128/jb.102.2.462-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafia F., Wilkinson R. F., Jr Growth of Ferrobacillus ferrooxidans on organic matter. J Bacteriol. 1969 Jan;97(1):256–260. doi: 10.1128/jb.97.1.256-260.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Hoare D. S. Acetate assimilation by Nitrobacter agilis in relation to its "obligate autotrophy". J Bacteriol. 1968 Mar;95(3):844–855. doi: 10.1128/jb.95.3.844-855.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., London J., Stanier R. Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967 Oct;94(4):972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabita R., Lundgren D. G. Utilization of glucose and the effect of organic compounds on the chemolithotroph Thiobacillus ferrooxidans. J Bacteriol. 1971 Oct;108(1):328–333. doi: 10.1128/jb.108.1.328-333.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudinger P. A., Kelly D. P. Reduced nicotinamide adenine dinucleotide oxidation by Thiobacillus neapolitanus and Thiobacillus strain C. J Bacteriol. 1968 May;95(5):1962–1963. doi: 10.1128/jb.95.5.1962-1963.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISHNIAC W., SANTER M. The thiobacilli. Bacteriol Rev. 1957 Sep;21(3):195–213. doi: 10.1128/br.21.3.195-213.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



