Abstract
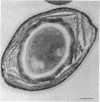
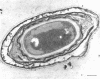
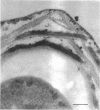
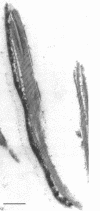
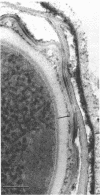
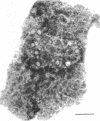
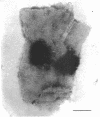
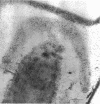
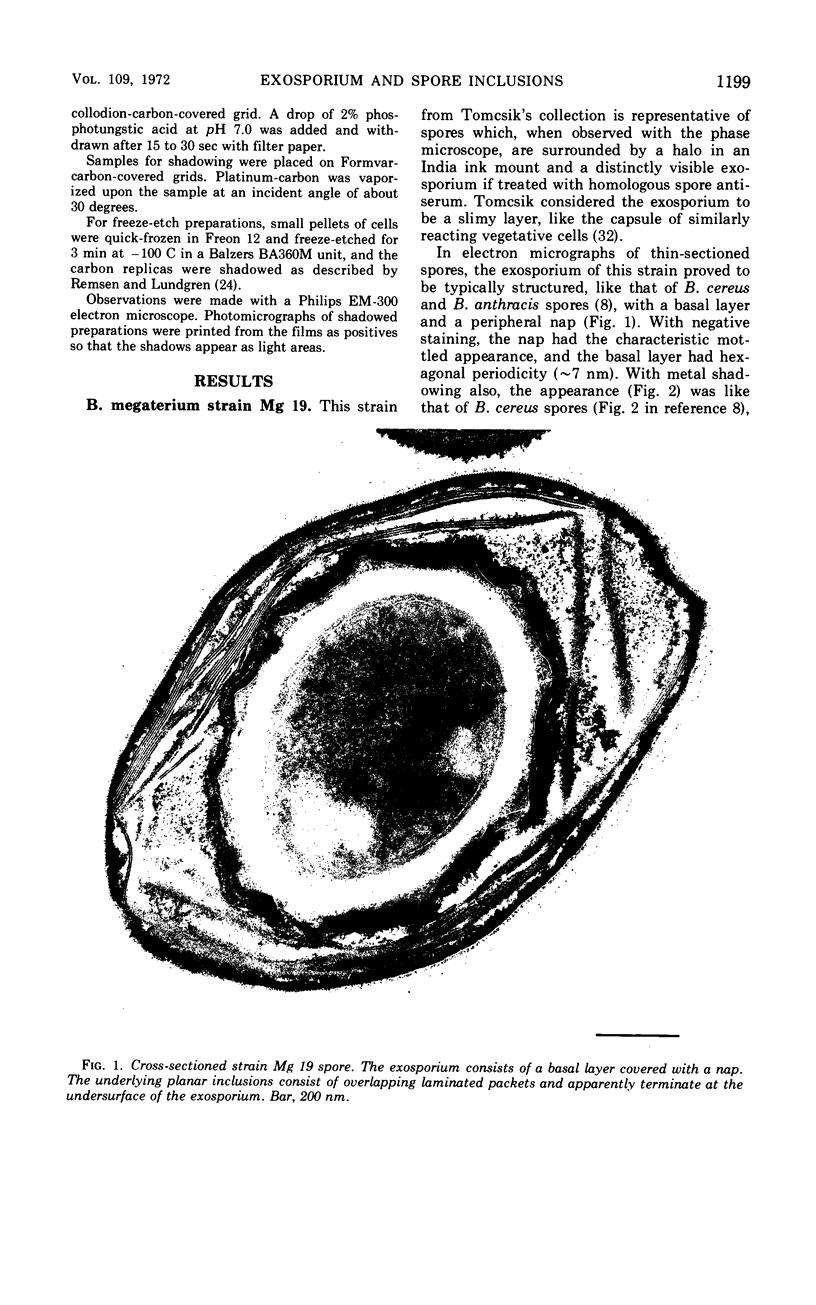
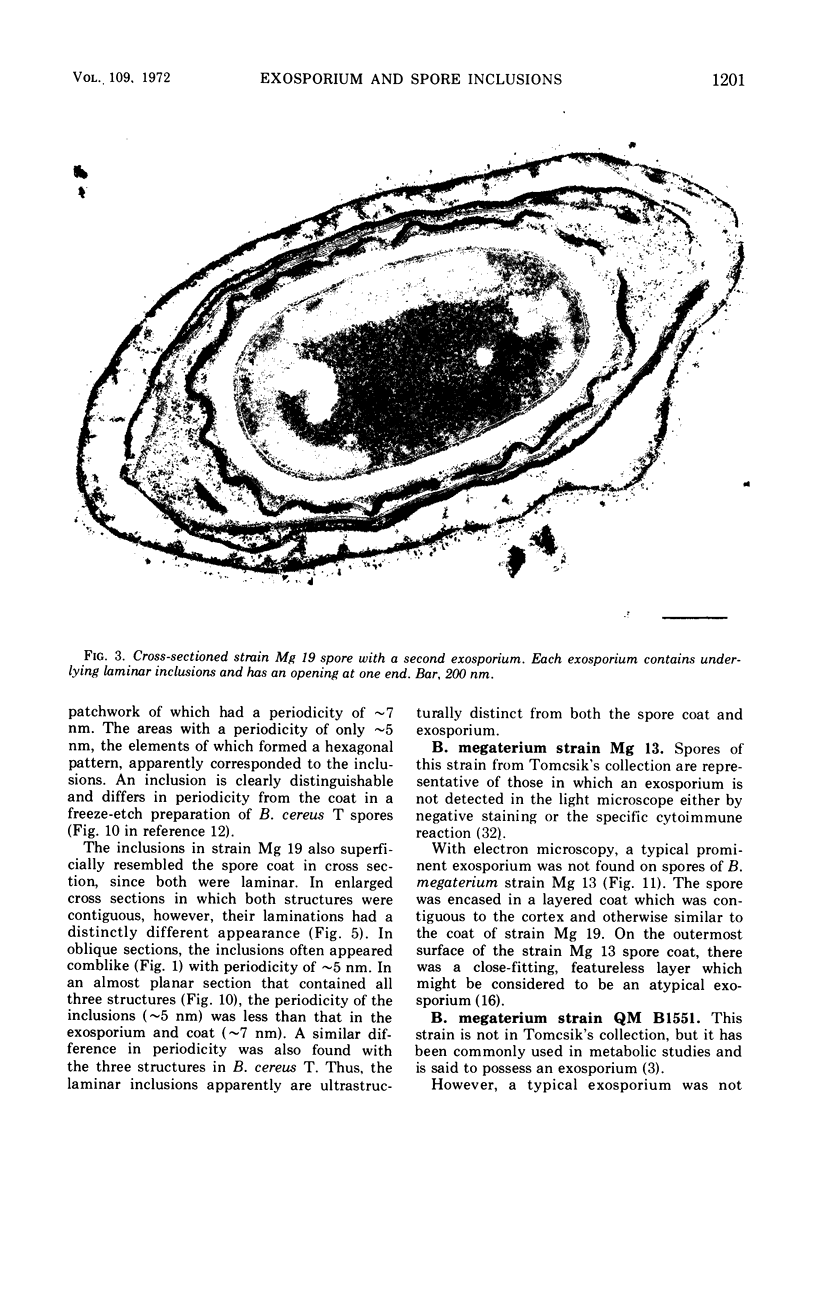
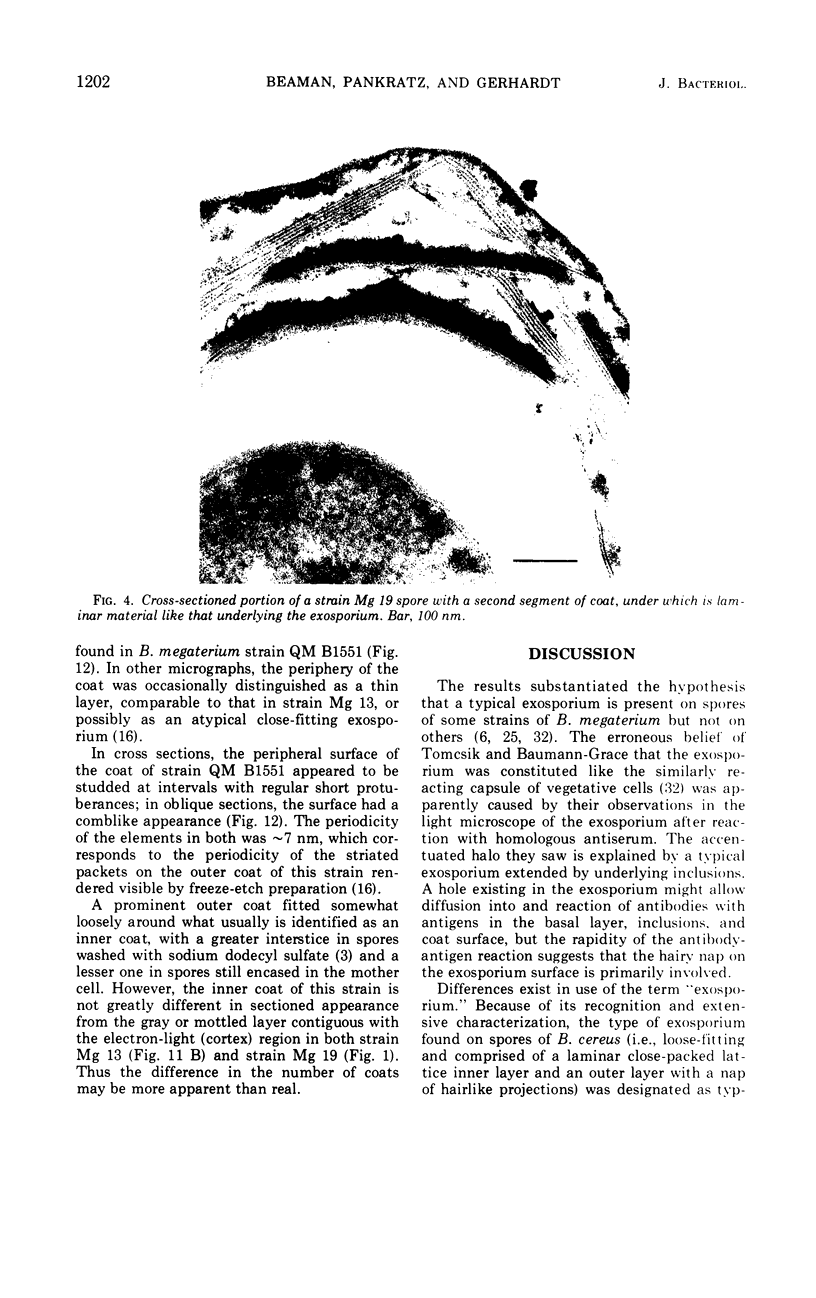
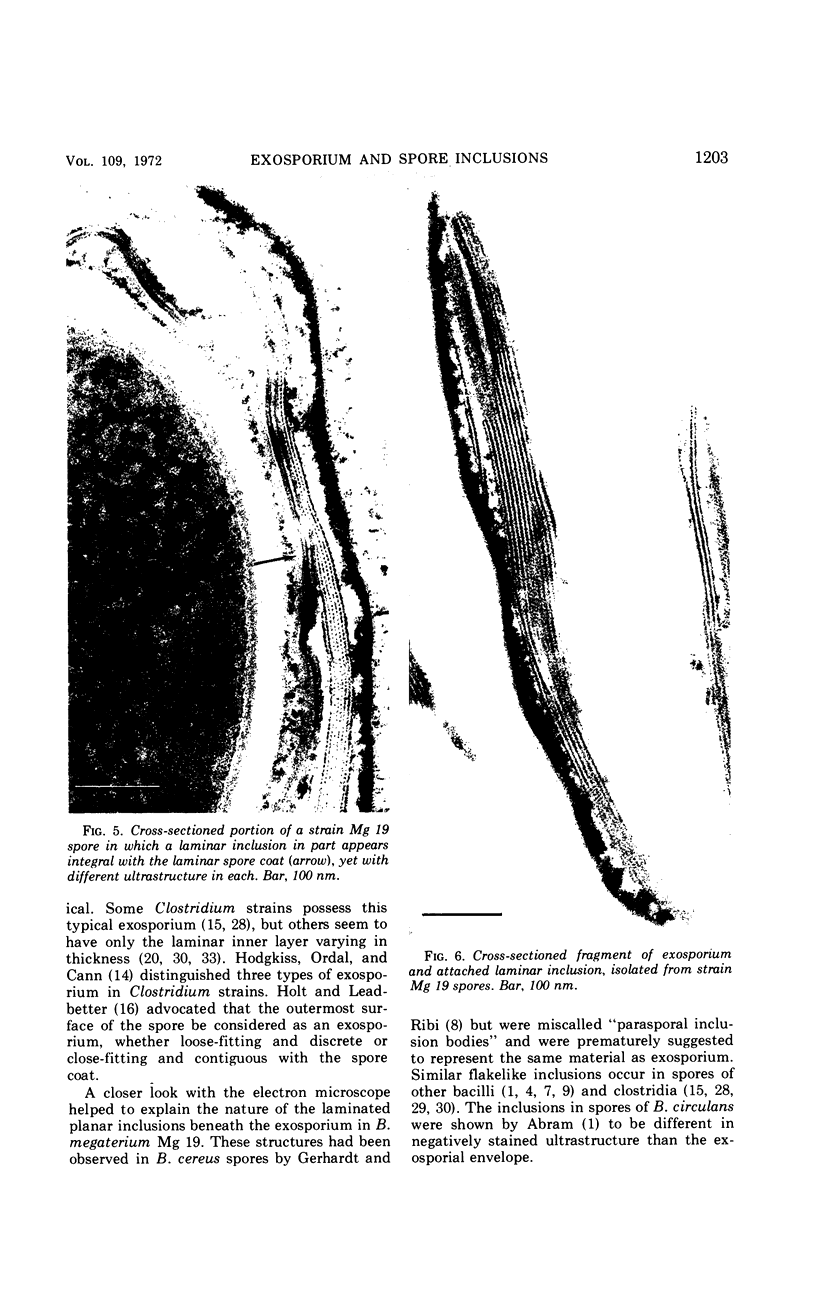
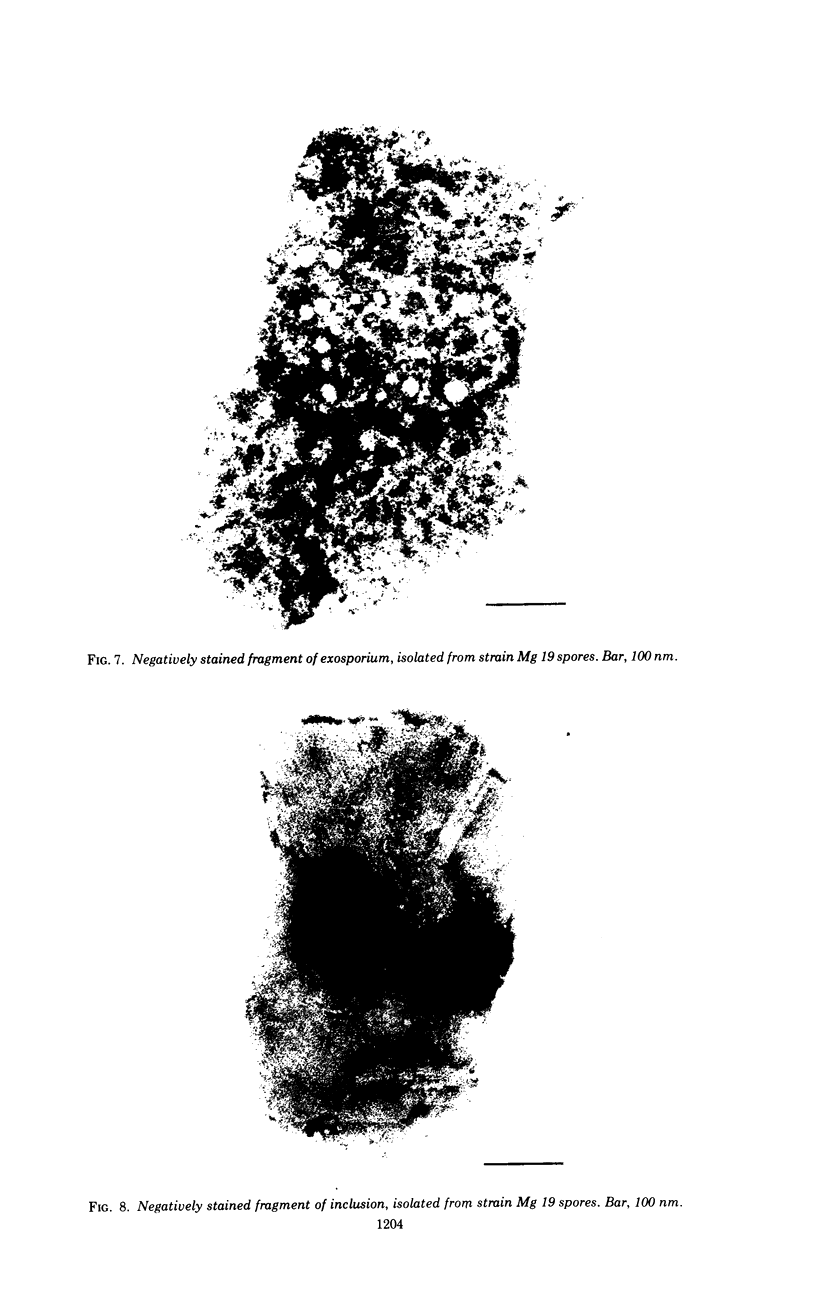
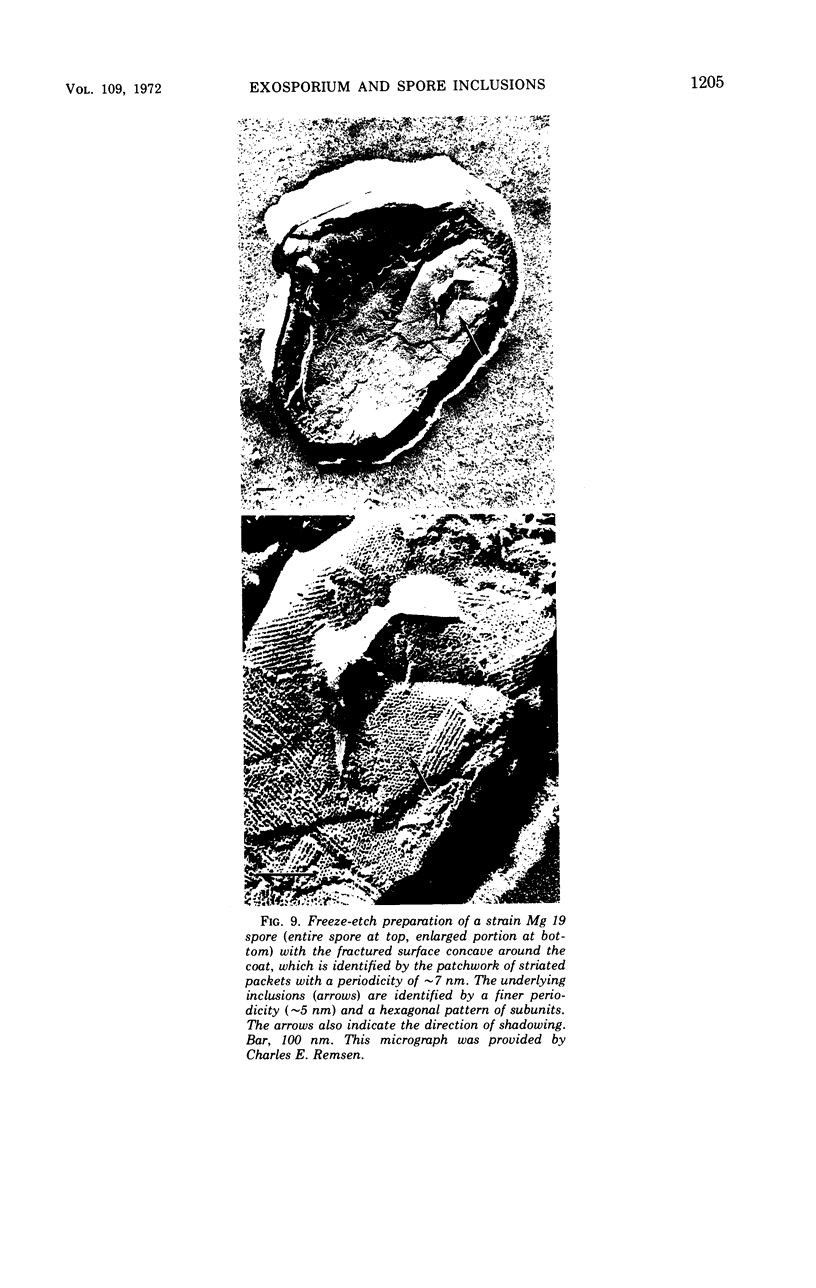
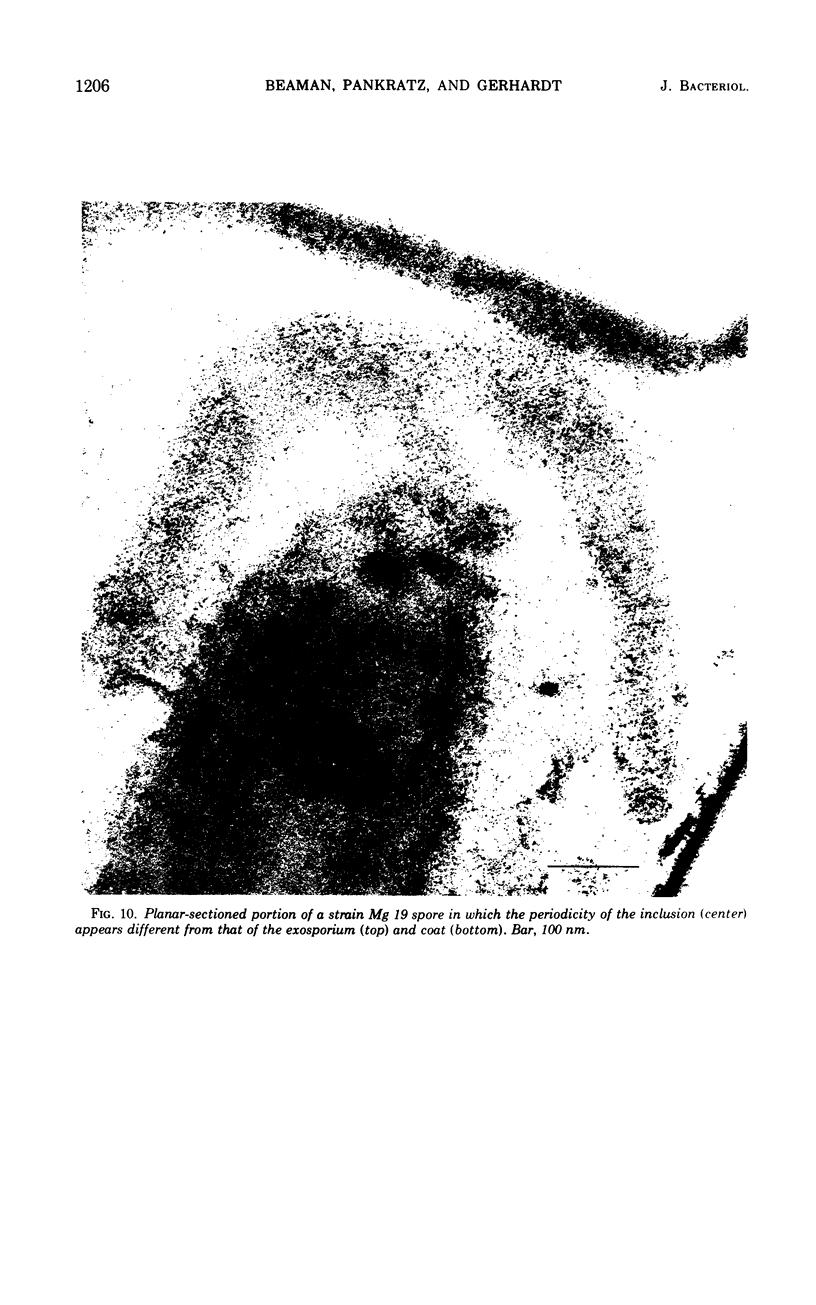
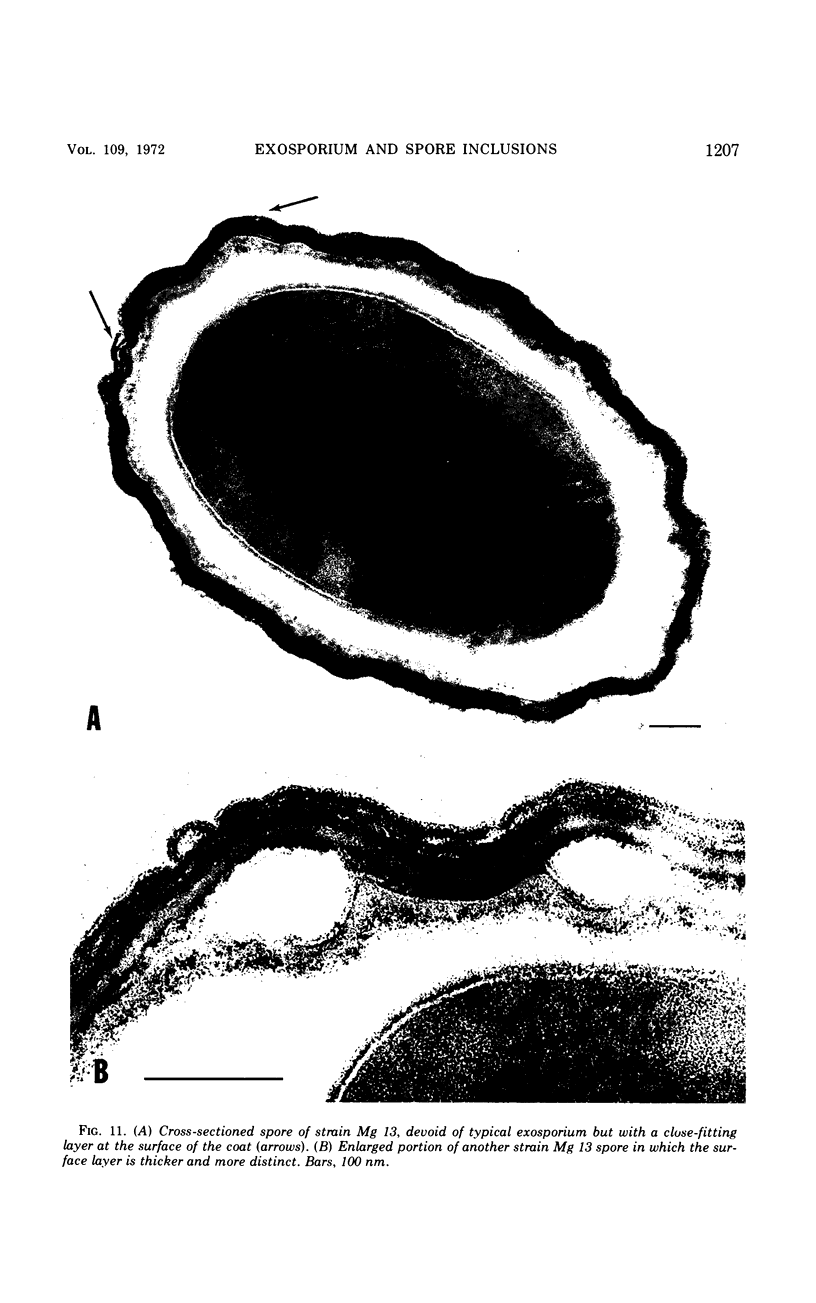
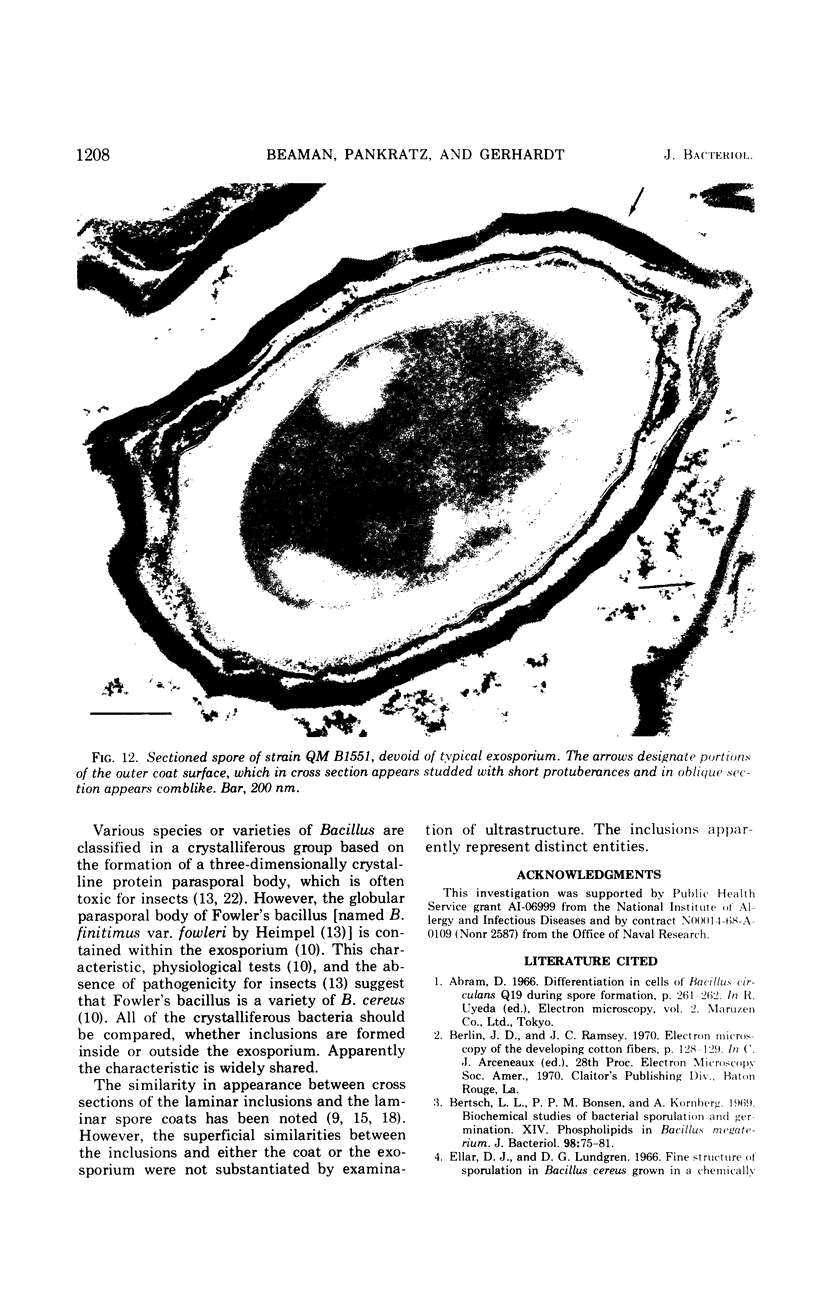
Spores of selected strains of Bacillus megaterium were prepared by various methods and examined with the electron microscope. An exosporium like that of B. cereus, with a nap and basal layer, was found in spores of a B. megaterium strain that reportedly contains a capsule-like exosporium. The exosporium occasionally appeared to be doubled or have an apical opening. Pili-like filaments were discerned on the surface. Beneath the exosporium were found large deposits of planar inclusions, which in cross section appeared laminated and in surface views consisted of a patchwork of striated packets with a periodicity of ∼5 nm. The inclusions were usually attached to the exosporium, but in ultrastructure they differed from both the exosporium and coat. In two other strains of B. megaterium, one or two coats occurred but a typical exosporium was not present.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellar D. J., Lundgren D. G. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J Bacteriol. 1966 Dec;92(6):1748–1764. doi: 10.1128/jb.92.6.1748-1764.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C., Young I. E. CYTOLOGICAL COMPARISON OF SPORES OF DIFFERENT STRAINS OF BACILLUS MEGATERIUM. J Bacteriol. 1959 Dec;78(6):755–764. doi: 10.1128/jb.78.6.755-764.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., RIBI E. ULTRASTRUCTURE OF THE EXOSPORIUM ENVELOPING SPORES OF BACILLUS CEREUS. J Bacteriol. 1964 Dec;88:1774–1789. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt P. Cytology of Bacillus anthracis. Fed Proc. 1967 Sep;26(5):1504–1517. [PubMed] [Google Scholar]
- HANNAY C. L. Fowler's bacillus and its parasporal body. J Biophys Biochem Cytol. 1961 Feb;9:285–298. doi: 10.1083/jcb.9.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- Hachisuka Y., Kojima K., Sato T. Fine filaments on the outside of the exosporium of Bacillus anthracis spores. J Bacteriol. 1966 Jun;91(6):2382–2384. doi: 10.1128/jb.91.6.2382-2384.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoo T., Conti S. F. Ultrastructural changes associated with activation and germination of Bacillus cereus T spores. J Bacteriol. 1971 Jan;105(1):361–368. doi: 10.1128/jb.105.1.361-368.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimpel A. M. A critical review of Bacillus thuringiensis var. thuringiensis Berliner and other crystalliferous bacteria. Annu Rev Entomol. 1967;12:287–322. doi: 10.1146/annurev.en.12.010167.001443. [DOI] [PubMed] [Google Scholar]
- Hodgkiss W., Ordal Z. J., Cann D. C. The morphology and ultrastructure of the spore and exosporium of some Clostridium species. J Gen Microbiol. 1967 May;47(2):213–225. doi: 10.1099/00221287-47-2-213. [DOI] [PubMed] [Google Scholar]
- Hoeniger J. F., Headley C. L. Ultrastructural aspects of spore germination and outgrowth in Clostridium sporogenes. Can J Microbiol. 1969 Sep;15(9):1061–1065. doi: 10.1139/m69-189. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. J., Roth I. L. Electron microscopic evidence for a double hair-like nap appearing at low frequency on Bacillus anthracis Sterne spores. Can J Microbiol. 1969 Oct;15(10):1247–1248. doi: 10.1139/m69-226. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz L. L., Beaman T. C., Gerhardt P. Chemical composition of exosporium from spores of Bacillus cereus. J Bacteriol. 1970 Jan;101(1):196–201. doi: 10.1128/jb.101.1.196-201.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pate J. L., Ordal E. J. The fine structure of Chondrococcus columnaris. 3. The surface layers of Chondrococcus columnaris. J Cell Biol. 1967 Oct;35(1):37–51. doi: 10.1083/jcb.35.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Remsen C., Lundgren D. G. Electron microscopy of the cell envelope of Ferrobacillus ferrooxidans prepared by freeze-etching and chemical fixation techniques. J Bacteriol. 1966 Dec;92(6):1765–1771. doi: 10.1128/jb.92.6.1765-1771.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonoff W. A., Hashimoto T., Conti S. F. Appendage development in Clostridium bifermentans. J Bacteriol. 1971 Apr;106(1):269–275. doi: 10.1128/jb.106.1.269-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonoff W. A., Hashimoto T., Conti S. F. Ultrastructural changes associated with germination and outgrowth of an appendage-bearing clostridial spore. J Bacteriol. 1970 Mar;101(3):1038–1045. doi: 10.1128/jb.101.3.1038-1045.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo L. M., Hohl H. R., Frank H. A. Ultrastructure of putrefactive anaerobe 3679h during sporulation. J Bacteriol. 1969 Sep;99(3):824–833. doi: 10.1128/jb.99.3.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D. Symposium on bacterial spores: I. Cytology of spore formation and germination. J Appl Bacteriol. 1970 Mar;33(1):1–12. doi: 10.1111/j.1365-2672.1970.tb05229.x. [DOI] [PubMed] [Google Scholar]