Abstract
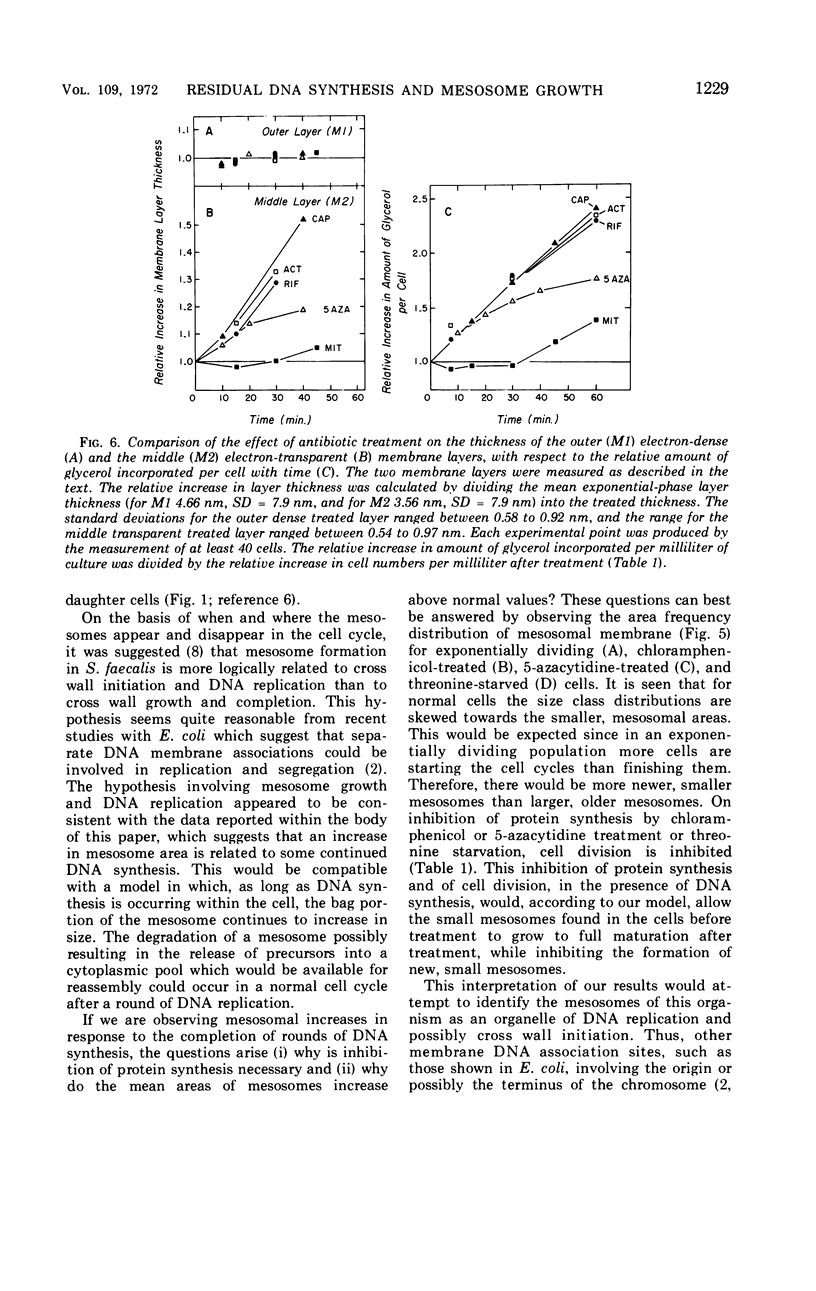
The application of quantitative electron microscopy to thin sections of cells of Streptococcus faecalis specifically inhibited for deoxyribonucleic acid (DNA), ribonucleic acid, and protein synthesis shows that septal mesosomes (i) increase in size when protein synthesis is inhibited by at least 80% while DNA synthesis proceeds at no less than 50% of the control rate and (ii) decrease in size when DNA synthesis is inhibited 50% or more during the initial 10 min of treatment. This indicates that fluctuations in mesosome size are dependent on the extent of DNA synthesis. The fluctuations in mesosome areas observed on treatment do not correlate with the kinetics of glycerol incorporation per milliliter of a culture. However, when glycerol incorporation is placed on a per cell basis, a strong correlation is observed between increases in (i) the thickness of the electron-transparent layer of the cytoplasmic membrane and (ii) the amount of glycerol incorporated per cell. It seems that the electron-transparent membrane layer may thicken to accommodate changes in lipid content when protein and lipid synthesis are uncoupled.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburg B. C., Suit J. C., Brinkley B. R. Ultrastructure of deoxyribonucleic acid-membrane associations in Escherichia coli. J Bacteriol. 1970 Oct;104(1):549–555. doi: 10.1128/jb.104.1.549-555.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambron R. T., Pieringer R. A. The metabolism of glyceride glycolipids. V. Identification of the membrane lipid formed from diglucosyl diglyceride in Streptococcus faecalis ATCC 9790 as an acylated derivative of glyceryl phosphoryl diglucosyl glycerol. J Biol Chem. 1971 Jul 10;246(13):4216–4225. [PubMed] [Google Scholar]
- Fuchs E., Hanawalt P. Isolation and characterization of the DNA replication complex from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):301–322. doi: 10.1016/0022-2836(70)90032-x. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Reinitiation of cell wall growth after threonine starvation of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):1175–1183. doi: 10.1128/jb.105.3.1175-1183.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Early changes in the ultrastructure of Streptococcus faecalis after amino acid starvation. J Bacteriol. 1970 Jul;103(1):244–253. doi: 10.1128/jb.103.1.244-253.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
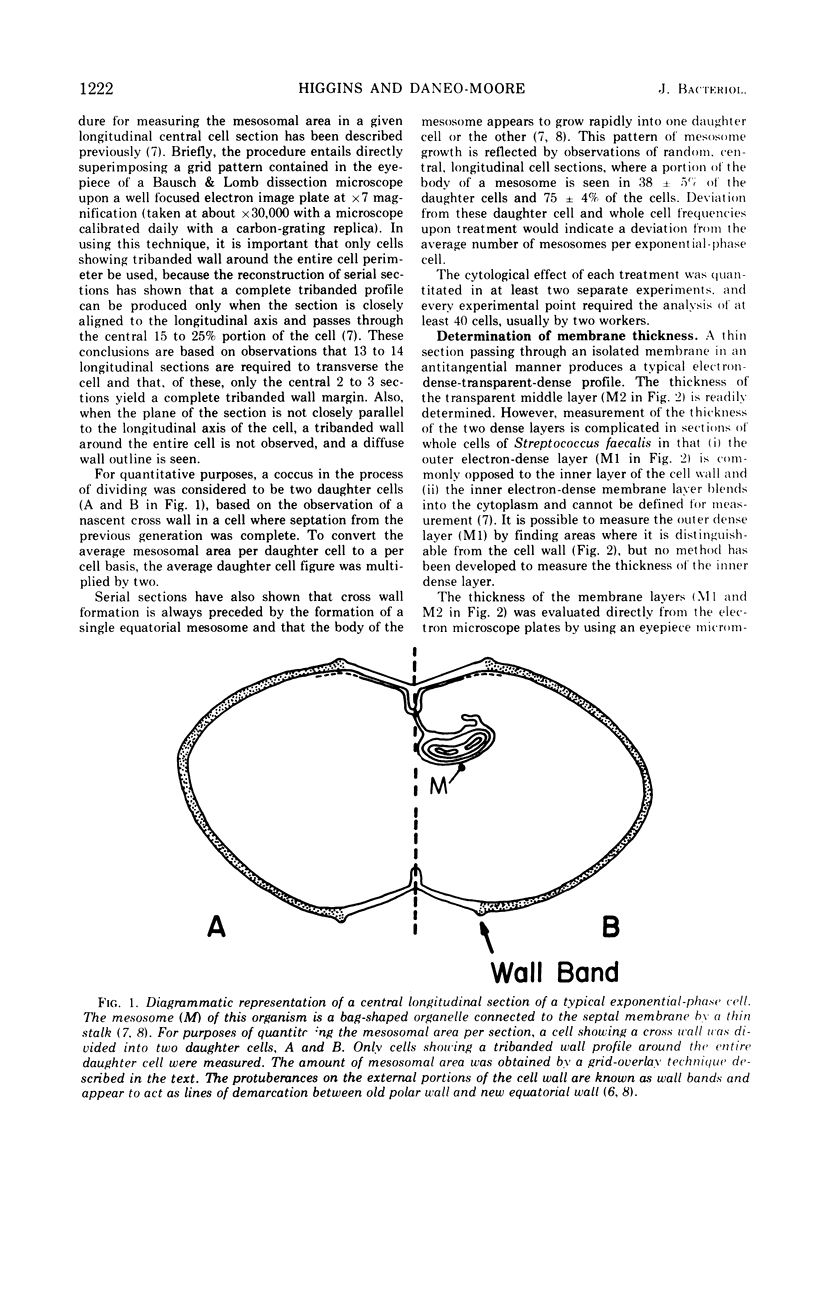
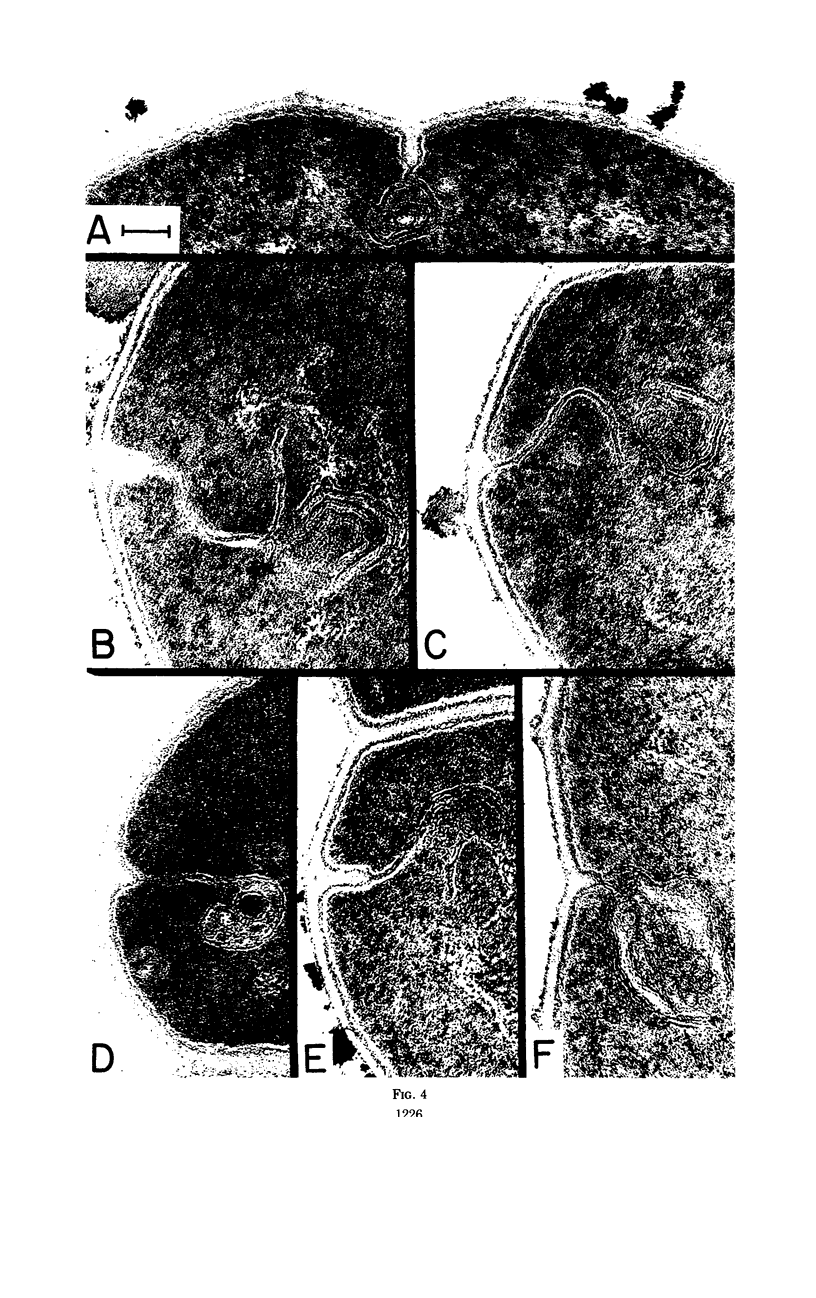
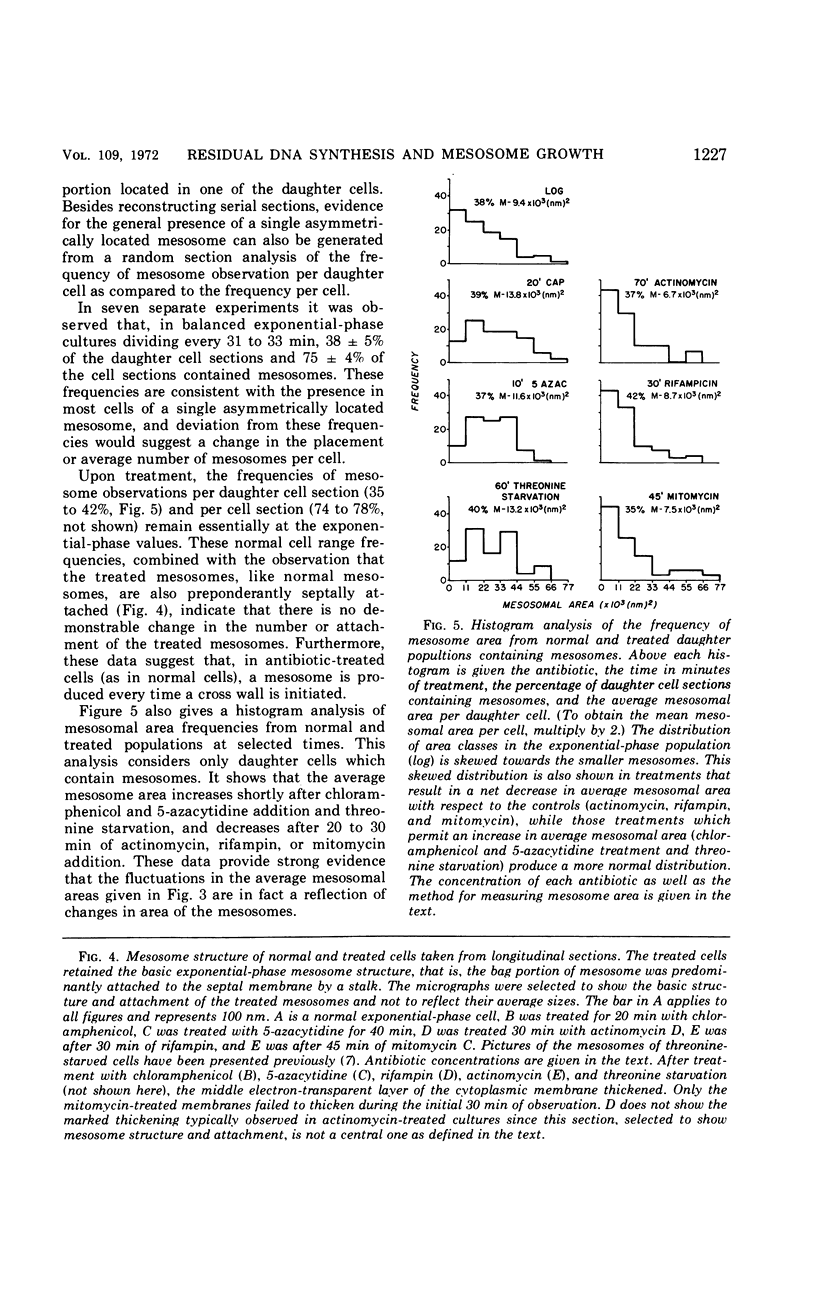
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Kahane I., Razin S. Synthesis and turnover of membrane protein and lipid in Mycoplasma laidlawii. Biochim Biophys Acta. 1969 Jun 3;183(1):79–89. doi: 10.1016/0005-2736(69)90131-x. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A. Association of the nucleus and the membrane of bacteria: a morphological study. Bacteriol Rev. 1968 Mar;32(1):39–54. doi: 10.1128/br.32.1.39-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R. Bacterial membranes. CRC Crit Rev Microbiol. 1971 May;1(1):161–197. doi: 10.3109/10408417109104480. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- TOENNIES G., ISZARD L., ROGERS N. B., SHOCKMAN G. D. Cell multiplication studied with an electronic particle counter. J Bacteriol. 1961 Dec;82:857–866. doi: 10.1128/jb.82.6.857-866.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOENNIES G., SHOCKMAN G. D., KOLB J. J. Differential effects of amino acid deficiencies on bacterial cytochemistry. Biochemistry. 1963 Mar-Apr;2:294–296. doi: 10.1021/bi00902a017. [DOI] [PubMed] [Google Scholar]
- Ziegler R. J., Daneo-Moore L. Effects of essential amino acid starvation in Streptococcus faecalis: structural change in the 50S ribosomal subunit. J Bacteriol. 1971 Jan;105(1):190–199. doi: 10.1128/jb.105.1.190-199.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]